It is widely accepted that people living with a diagnosis of cancer experience higher levels of psychological distress than the general population.1,2 However, cancer is a heterogeneous condition, and recent evidence suggests that although high levels of psychological distress are seen at the time of diagnosis and treatment3 and with advanced disease,4 long-term survivors may have similar rates of anxiety and depression to the general population.5 There is a lack of reliable evidence on the joint contributions of the diagnosis of cancer, current treatment and functional impairment to psychological distress.
The 45 and Up Study is a large-scale study of healthy ageing of men and women from the general population of New South Wales, Australia. It is described in detail elsewhere.6 Briefly, individuals aged 45 years and over were sampled from the Medicare Australia database, with oversampling of rural residents and older people. Participants joined the study by completing a postal questionnaire and providing written consent for follow-up. Recruitment began on 1 February 2006. These analyses relate to 89 574 participants who joined the study up to 30 April 2008 and had valid Kessler Psychological Distress Scale (K10) scores.7,8
All the variables we examined in this study were from self-reported data from the 45 and Up Study questionnaire (available at http://www.45andUp.org.au), apart from remoteness of residence, which was derived from the Accessibility Remoteness Index of Australia Plus (ARIA+)9 score for each participant’s postcode. Relevant variables were grouped according to the categories in Box 1 and Box 2.
The outcome measure used in these analyses was symptoms of psychological distress over the previous 4 weeks,10 as measured by the K10.7 Scores on the K10 range from 10 (no distress) to 50 (severe distress), and scores of 22 and above were considered indicative of high levels of psychological distress.11
Overall, 7.5% of participants had K10 scores indicating high levels of psychological distress. The prevalence of high psychological distress generally decreased with increasing age, was significantly higher in those reporting lower educational qualifications, lower income, a language other than English spoken at home (Box 1) and current smoking (Box 2), and varied significantly according to work status (Box 1). The risk of high psychological distress was six to eight times greater in people needing help with daily tasks and in those with functional impairment, than in those without such disabilities (Box 2).
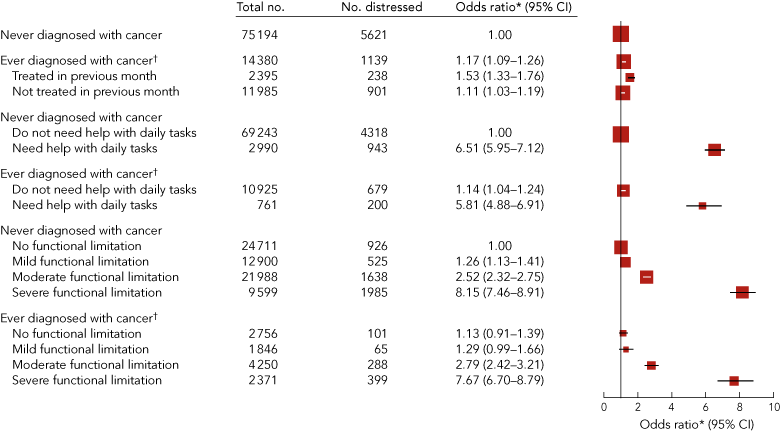
Compared with individuals without cancer, the adjusted OR for a high level of psychological distress was 1.17 (1.09–1.26) in individuals reporting having had cancer apart from non-melanoma skin cancer, 1.53 (1.33–1.76) for those reporting cancer treatment in the previous month and 1.11 (1.03–1.19) for those reporting cancer without treatment in the previous month (Box 3; P[heterogeneity] < 0.001 for recent treatment v not recent treatment, among cancer survivors). Additional adjustment for smoking status, alcohol consumption, physical activity and language other than English spoken at home did not change these ORs materially (ie, the OR changed by < 5%) so these factors were not included in the final model.
Using individuals with neither cancer nor disability as the reference group, the adjusted OR for psychological distress was 6.51 (5.95–7.12) in those reporting needing help with daily tasks but no cancer, 1.14 (1.04–1.24) in those with cancer but not needing help and 5.81 (4.88–6.91) in those with cancer and needing help with daily tasks (Box 3). A similar pattern of increasing psychological distress with increasing degree of functional limitation was seen, independent of the diagnosis of cancer (Box 3). There was no significant difference in the effect of functional limitation on psychological distress according to cancer survivor status (P[interaction] = 0.60), and, although the relationship between needing help with daily tasks and psychological distress was significantly attenuated in cancer survivors compared with cancer-free individuals (P[interaction] = 0.01), this difference did not appear to be clinically meaningful (Box 3). When the OR for psychological distress in people with cancer treated in the previous month versus those without cancer was further adjusted for level of functional limitation, it decreased from 1.53 to 1.16 (1.00–1.34), confirming that functional limitation, was responsible for a substantial proportion of the overall effect.
High psychological distress did not differ significantly between men without cancer and those reporting prostate cancer, regardless of treatment status (Box 4). Men reporting recent treatment for melanoma or for “other cancers” (ie, other than prostate cancer, melanoma and non-melanoma skin cancer) had significantly increased distress, but this was of borderline significance in those with melanoma (Box 4). For women, there was no significant difference in psychological distress between those without cancer and those reporting breast cancer or melanoma cancer, regardless of recent treatment status. There was a significantly increased risk of high psychological distress among those with “other” cancers, both with and without recent treatment and with or without disability. Men and women ever diagnosed with non-melanoma skin cancer had a small, marginally significant reduction in the risk of psychological distress (Box 4). The OR for psychological distress related much more strongly to disability than to site-specific cancer diagnosis (Box 4).
A diagnosis of cancer is associated with increased psychological distress.2 Other studies have shown that physical disability is one of the most consistent predictors of psychological distress, in the general population.2,12 The interaction between mental health and disability is complex; depression predicts the onset and progression of both social and physical disability, and psychological distress can contribute to functional impairment.13 The measure of disability that we used, relating to needing help with daily tasks, does not specify the cause of the disability and therefore includes disability resulting from mental health problems. The measure of functional capacity relates predominantly to physical disability. The few studies to investigate the association between disability and psychological distress among people with cancer have tended to focus on cancers at specific sites and, consistent with our findings, have observed functional impairment to be the most important predictor of psychological distress in patients with lung cancer14 and ovarian cancer.15 One study found increased depression and anxiety in cancer survivors who were recipients of disability-support pensions.5 Other factors, such as pain and side effects of treatment, may also play a role. As with previous studies, our findings show recent cancer diagnosis and treatment to be associated with increased distress3,4,15 and that some increase in distress may persist in the long term.16,17
The association between psychological distress and cancer may also vary according to other factors such as the cancer type, stage, grade and prognosis. In a previous study of patients with predominantly newly diagnosed cancer, psychological distress was highest for cancers associated with a poor prognosis, such as those of the lung, pancreas, brain and liver.4 Although we were able to present new insights showing minimal effects on psychological distress of a diagnosis of breast cancer, prostate cancer, melanoma and non-melanoma skin cancer, practical constraints prevented us from exploring this in depth for other cancer types. The increased risk of distress in those with “other” cancer types may reflect the proportion of tumours associated with a poor prognosis in this group; it would be of interest to explore this further, using linkage to cancer registrations among participants.
The large size, population-based nature and wide age range of the 45 and Up Study allowed us to examine both main effects and interactions between groups of interest. The study uses a well established and validated measure of psychological distress,10 incorporating both anxiety and depression. The K10 does not permit separate consideration of anxiety or depression, because the scores on individual items are highly correlated with each other and with the total score (overall alpha, 0.88). Nor are we able to determine whether the distress, or disability, is acute or chronic in nature. The 45 and Up Study questionnaire has two measures of general functional impairment, but does not allow direct attribution of disability to cancer. These analyses use cross-sectional data, so it is not possible to establish the temporal relationship between the diagnosis of cancer and the occurrence of psychological distress. However, our findings regarding recency of diagnosis and the role of disability are reassuring, as they suggest intuitively reasonable temporal and aetiological relationships that are consistent with the evidence to date.
The 45 and Up Study is a population-based cohort study, with a response rate of 18%,6 which is in keeping with other cohort studies of this nature. It is important to note that both theoretical18 and empirical19 evidence shows that representativeness is not necessary for generating reliable estimates of relative risk based on internal comparisons from within such a cohort, including those relating to psychological distress. Cancer survivors included in the 45 and Up Study may not be representative of cancer survivors more broadly. There may have been an under-representation of those with aggressive disease, cancers with a poor prognosis or advanced disease, and those on current treatment, including rural residents attending urban-based treatment facilities; this potential bias would generally cause underestimation of the association between cancer and psychological distress.
The cancers included in our study are based on self-report. Although this provides a good general indication of cancer status, especially for breast and prostate cancer, the validity of such data varies according to cancer site and other factors;20-23 results on non-melanoma skin cancer in particular should be regarded with caution. As the main issue with self-reported cancer relates to reduced sensitivity for some cancer sites, and as specificity is generally good, any resulting bias would tend to lead to more conservative estimates of effect in relation to psychological distress.
1 Psychological distress in relation to demographic factors for 89 574 participants in the 45 and Up Study
Odds ratio adjusted for age, sex, income and education (95% CI) |
|||||||||||||||
2 Psychological distress in relation to lifestyle and disability factors for 89 574 participants in the 45 and Up Study
Odds ratio adjusted for age, sex, income and education (95% CI) |
|||||||||||||||
3 Odds ratios for high psychological distress according to cancer diagnosis, recent treatment and disability
 |
- Emily Banks1
- Julie E Byles2
- Richard E Gibson2
- Bryan Rodgers3
- Isabel K Latz1
- Ian A Robinson2
- Anna B Williamson4
- Louisa R Jorm4,5
- 1 National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT.
- 2 Centre for Gender, Health and Ageing, School of Medicine and Public Health, University of Newcastle, Newcastle, NSW.
- 3 Australian Demographic and Social Research Institute, Australian National University, Canberra, ACT.
- 4 The Sax Institute, Sydney, NSW.
- 5 School of Medicine, University of Western Sydney, Sydney, NSW.
We thank the men and women participating in the 45 and Up Study. The 45 and Up Study is managed by The Sax Institute in collaboration with major partner Cancer Council New South Wales, and partners: the National Heart Foundation of Australia (NSW Division); NSW Health; beyondblue: the national depression initiative; Ageing, Disability and Home Care, Department of Human Services NSW; and UnitingCare Ageing. The work for this article was supported by beyondblue: the national depression initiative and National Health and Medical Research Council (NHMRC) grant 402810. Emily Banks and Bryan Rodgers are supported by the NHMRC.
None identified.
- 1. Evans DL, Charney DS, Lewis L, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry 2005; 58: 175-189.
- 2. Pfaff JJ, Draper BM, Pirkis JE, et al. Medical morbidity and severity of depression in a large primary care sample of older Australians: the DEPS-GP project. Med J Aust 2009; 190 (7 Suppl): S75-S80. <MJA full text>
- 3. Breen SJ, Baravelli CM, Schofield PE, et al. Is symptom burden a predictor of anxiety and depression in patients with cancer about to commence chemotherapy? Med J Aust 2009; 190 (7 Suppl): S99-S104. <MJA full text>
- 4. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology 2001; 10: 19-28.
- 5. Boyes AW, Girgis A, Zucca AC, Lecathelinais C. Anxiety and depression among long-term survivors of cancer in Australia: results of a population-based survey. Med J Aust 2009; 190 (7 Suppl): S94-S98. <MJA full text>
- 6. 45 and Up Study Collaborators. Cohort profile: the 45 and Up Study. Int J Epidemiol 2008; 37: 941-947.
- 7. Kessler R, Mroczek D. Final version of our non-specific psychological distress scale [memo dated 3/10/94]. Ann Arbor, Mich: Survey Research Center of the Institute for Social Research, University of Michigan, 1994.
- 8. Kessler RC, Andrews G, Colpe LJ, Hiripi E. Short screening scales to monitor population prevalences and trends in nonspecific psychological distress. Psychol Med 2002; 32: 959-976.
- 9. Australian Institute of Health and Welfare. Rural, regional and remote health: a guide to remoteness classifications. Canberra: AIHW, 2004. (AIHW Cat. No. PHE 53.) http://www.aihw.gov.au/publications/index.cfm/title/9993 (accessed Jun 2010).
- 10. Andrews G, Slade T. Interpreting scores on the Kessler Psychological Distress Scale (K10). Aust N Z J Public Health 2001; 25: 494-497.
- 11. Australian Bureau of Statistics. National Survey of Mental Health and Wellbeing: summary of results, 2007. Canberra: ABS, 2008. (ABS Cat. No. 4326.0.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/mf/4326.0 (accessed Nov 2009).
- 12. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 2003; 160: 1147-1156.
- 13. Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet 2007; 370: 859-877.
- 14. Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol 2000; 18: 893-903.
- 15. Bodurka-Bevers D, Basen-Engquist K, Carmack CL, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol 2000; 78: 302-308.
- 16. Wenzel L, DeAlba I, Habbal R, et al. Quality of life in long-term cervical cancer survivors. Gynecol Oncol 2005; 97: 310-317.
- 17. Deimling GT, Kahana B, Bowman KF, Schaefer ML. Cancer survivorship and psychological distress in later life. Psychooncology 2002; 11: 479-494.
- 18. Ponsonby A-L, Dwyer T, Couper D. Is this finding relevant? Generalisability and epidemiology. Aust N Z J Public Health 1996; 20: 54-56.
- 19. Mealing N, Banks E, Jorm L, et al. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol 2010; 10: 26.
- 20. Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol 1998; 147: 969-977.
- 21. Freedman DM, Sigurdson AJ, Doody MM, et al. Comparison between cancers identified by state cancer registry, self-report, and death certificate in a prospective cohort study of US radiologic technologists [letter]. Int J Epidemiol 2006; 35: 495-497.
- 22. Parikh-Patel A, Allen M, Wright WE. Validation of self-reported cancers in the California Teachers Study. Am J Epidemiol 2003; 157: 539-545.
- 23. Schrijvers C, Stronks K, van de Mheen DH, et al. Validation of cancer prevalence data from a postal survey by comparison with cancer registry records. Am J Epidemiol 1994; 139: 408-414.





Abstract
Objective: To investigate whether the observed elevated levels of psychological distress in cancer survivors relate specifically to aspects of cancer diagnosis, to treatment or to disability.
Design, participants and setting: Self-reported questionnaire data on demographic, health and lifestyle factors and mental health from 89 574 Australian men and women aged 45 years or older, sampled from the Medicare database for the 45 and Up Study from 1 February 2006 to 30 April 2008. Logistic regression was used to examine the risk of high levels of psychological distress in relation to cancer diagnosis and disability, adjusting for age, sex, income and education.
Main outcome measure: High psychological distress (Kessler Psychological Distress Scale score ≥22).
Results: Overall, 7.5% of participants had high levels of psychological distress. Among cancer survivors, the median time since diagnosis was 7.3 years. Compared with people without cancer, the odds ratios (95% CIs) for psychological distress were: 1.17 (1.09–1.26) in people reporting having had any cancer apart from non-melanoma skin cancer; 1.34 (1.08–1.67) in those with cancer diagnosed in the previous year; 1.53 (1.33–1.76) for those reporting treatment for cancer in the previous month and 1.11 (1.03–1.19) for those with cancer but without recent treatment. Using individuals with neither cancer nor disability as the reference group, the adjusted odds ratio (95% CI) for psychological distress was 6.51 (5.95–7.12) in those reporting significant disability but no cancer, 1.14 (1.04–1.24) in those without disability but with cancer and 5.81(4.88–6.91) in those with both cancer and disability.
Conclusion: The risk of psychological distress in individuals with cancer relates much more strongly to their level of disability than it does to the cancer diagnosis itself.