There has been considerable improvement in survival from haematological malignancies in children over the past three decades,1,2 but slower improvement for adolescents and young adults.3-6 The reasons for this difference are complex and include a lack of specialist care guidelines, differences in cancer biology, differences in chemotherapy pharmacokinetics, and a relative lack of clinical trials relevant to these older age groups.7-9
Our aim was to determine whether there has been any improvement in this “survival gap” for haematological malignancies in adolescents and young adults compared with children in Australia. We used a population-based, national dataset to determine and compare trends for 5-year survival from the main haematological malignancies in these age groups.
The Australian Cancer Database (ACD), held by the Australian Institute of Health and Welfare’s National Cancer Statistics Clearing House, contains cancer incidence and mortality data for all invasive cancers (excluding non-melanoma skin cancer) diagnosed in Australia from 1982 onwards. The ACD data are routinely compiled from cancer data from individual Australian state and territory registries, which collect information on patient demographics, primary tumour site and tumour morphology from hospital, pathology, radiotherapy and physicians’ records. Vital status is determined through linkages to state or territory registries of births, deaths and marriages, and to the National Death Index. Malignancies in the ACD are classified according to the International classification of diseases for oncology, third edition (ICD-O-3).
We divided these data into four groups: acute lymphoblastic leukaemia (ALL), acute myeloid leukaemia (AML), Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). Leukaemias and lymphomas were defined on the basis of the International classification of childhood cancer, third edition (ICCC-3),10 with some modifications for ALL and NHL to allow comparison with other Australian data. Myelodysplastic and myeloproliferative disorders, lymphoreticular neoplasms and leukaemias other than ALL and AML were excluded.
Data were analysed according to the three age groups of children (0–14 years), adolescents (15–19 years) and young adults (20–29 years); and four periods of diagnosis (1982–1989, 1990–1994, 1995–1999 and 2000–2004). Follow-up data for vital status were available up to 31 December 2006. Five-year overall survival for each period of diagnosis was calculated for each age group and type of malignancy using Kaplan–Meier estimates. All analyses were conducted in Stata, version 10.1 SE (StataCorp, College Station, Tex, USA).
As the ACD data were routine, aggregated, de-identified incidence and survival data with no possibility of re-identification, ethics approval was not required.
During the years 1982–2004, 13 015 people aged up to 29 years were diagnosed with primary leukaemia or lymphoma in Australia. We excluded from our analysis 44 people whose malignancy was reported to the ACD only on a death certificate, and one person for whom the follow-up time was unavailable. Also excluded were 621 people (4.8%) diagnosed with a leukaemia other than ALL or AML, 256 (2.0%) with a myelodysplastic or myeloproliferative disorder, and 178 (1.4%) with a lymphoreticular neoplasm. This left a dataset with records for 11 915 patients: 5355 children, 1970 adolescents and 4590 young adults. More than half (57.8%) were male (Box 1).
Incidence rates of ALL were higher among children (63.7%) than adolescents (22.9%) or young adults (8.9%). Proportions with AML were similar across the three age groups (children, 12.7%; adolescents, 13.6%; young adults, 14.0%). Both types of lymphoma were proportionately more common in adolescents and young adults than children (NHL: children, 14.8%; adolescents, 22.5%; young adults, 31.7%; and HL: children, 8.8%; adolescents, 41.0%; young adults, 45.4%).
Of the 11 915 patients, 8516 (71.5%) were still alive on the census date (31 December 2006). Median follow-up time for the surviving patients was 11 years (range, 2–25 years).
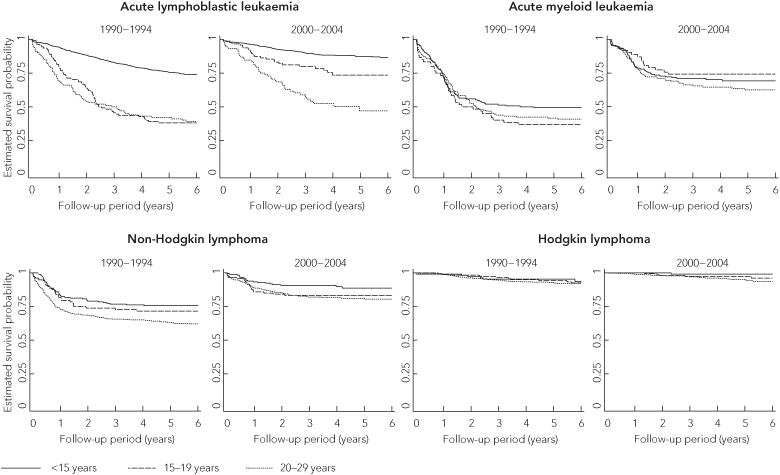
Considerable improvements in survival were seen for leukaemia and lymphoma in all age groups, particularly since 1995 (Box 2, Box 3). The most marked improvement was for adolescent ALL (73.6% in 2000–2004 v 39.6% in 1982–1989), such that the arithmetic difference in 5-year survival compared with children reduced from 34.1% to 13.9%. Although there was also an improvement in survival for young adults with ALL, a large difference in survival compared with children (about 40%) remained for this age group.
Historically, survival from AML (in all age groups) has been lower than survival from ALL, but 5-year survival for adolescents diagnosed in 2000–2004 was similar for AML and ALL. For young adults diagnosed in 2000–2004, 5-year survival was better for AML (62.5%) than for ALL (47.1%). In children, despite marked improvements in survival from AML (69.2% in 2000–2004 v 38.9% in 1982–1989), it remained considerably lower than survival from ALL (87.5% in 2000–2004).
Survival from lymphoma was generally better than survival from leukaemia. Survival from HL was remarkably good, with more than 94% of patients diagnosed with HL from 1995 onwards surviving 5 years. Survival from NHL improved considerably in all age groups from 1982–1989 to 2000–2004, but remained significantly poorer than survival from HL (Box 2).
Although international comparisons are hindered by differences in diagnostic criteria, time periods and analysis methods, the available data show that survival in Australia is currently at least as good as in Europe and the United States (Box 4).11-13
These population-based data confirm an improvement in survival from haematological malignancies across all three age groups of children, adolescents and young adults in Australia. However, a survival gap remains for the older age groups compared with children, especially for young adults with ALL.
The large differences persisting for ALL may be due to inherent differences in its biology in young adults compared with children, but it is also possible that the gap might be narrowed through alternative models of care or improved treatment regimens. In childhood ALL, the Berlin–Frankfurt–Münster (BFM) approach, which involves intensive induction and intensification chemotherapy associated with significant and often prolonged myelosuppression, has been adopted by most American, European and Australian paediatric cooperative groups, with excellent results.14 In many adult centres, alternative strategies have been applied for young adults and adolescents with ALL. Less complex, lower-morbidity induction regimens, often focusing on the role of allogeneic or autologous haematopoietic stem cell transplantation (HSCT), have been used. As overall outcomes remained poor, many patients were regarded as having sufficiently high risk of relapse to warrant HSCT in first remission. It is unclear to what extent allogeneic transplantation would improve outcomes in young adults after a BFM-style induction and consolidation treatment.14
A recent US retrospective study compared outcomes in 16–20-year-old patients with ALL who were treated on either the Children’s Cancer Group (CCG) protocol or the adult Cancer and Leukemia Group B (CALGB) protocol between 1988 and 2001. The complete remission rates were identical for both regimens, but the survival outcome was significantly poorer for the CALGB protocol than the CCG protocol (7-year event-free survival, 34% v 63%).14 An Intergroup trial involving adolescents and young adults with ALL currently underway in the US will include prospective evaluation of biological prognostic factors and treatment adherence by both patients and physicians.6
The improved 5-year survival for our adolescent ALL cohort in Australia (from 40% to 74%) may be due to either the adaptation of paediatric protocols or the use of elective allogeneic transplantation. There is anecdotal evidence that a number of paediatric centres treat adolescents up to the age of 17 years, and some adult centres have already adopted a BFM approach for young adults. The improvement in 5-year survival for young adults with ALL (from 31% to 47%) seems likely to be associated with improved outcomes of allogeneic HSCT, as there have been few changes in chemotherapy treatment approach for this group. Potential significant late sequelae related to the use of total body radiation and acute or chronic graft-versus-host disease encouraged paediatricians to move away from allogeneic HSCT for treating ALL, and it is essential that the role of this high-morbidity procedure is clearly defined in the context of optimal chemotherapy.15-18
The improvement in 5-year survival for children with AML (from 39% to 69%) occurred during a period when the use of allogeneic HSCT improved results in some subgroups, but the improvement is likely to be due to application of higher-dose induction and consolidation regimens. A similar improvement was seen in adolescents and, to a slightly lesser extent, in young adults. The lack of difference in outcome across the age groups reflects a recent general consensus in treatment approach across the age range and perhaps less biological heterogeneity of AML.
Improvement in 5-year survival for children with lymphoma was mirrored by a similar improvement in both the other age groups, ranging from 17% to 20% for NHL. Of the four haematological malignancies considered, NHL has the greatest heterogeneity of pathological subtypes. In children, the most common condition is Burkitt lymphoma (40%), followed by lymphoblastic lymphoma (30%), large B-cell lymphoma (20%) and anaplastic large cell lymphoma (10%). In adolescents, large B-cell lymphoma and mediastinal large B-cell lymphoma are more common; and in young adults, diffuse large B-cell, low-grade follicular lymphoma, marginal zone B-cell lymphoma, and peripheral T-cell lymphoma become more prominent.18-20 However, outcome in relation to pathological subtype of NHL was not available in this dataset.
It is now generally accepted that a paediatric approach should be applied to adolescents and young adults with Burkitt lymphoma or B-cell leukaemia.21 The role of the monoclonal antibody rituximab in treating high-grade B-cell lymphoma in children remains unclear. In adults with large B-cell lymphoma, the use of higher-dose intensive chemotherapy combined with rituximab now achieves excellent results.22 Treatment for the rarer anaplastic large-cell lymphoma and precursor lymphoblastic lymphoma are less standardised, and published series have had small patient numbers.23,24
In treating HL, there has been a move away from multiple alkylating-agent regimens combined with involved- or extended-field radiotherapy. In children and young adults, very high cure rates are achieved with hybrid regimens combining sterilising and non-sterilising drugs and involved-field radiation.25 The introduction of positron emission tomography imaging allowed increased confidence to omit radiation where complete remission has been achieved. There are few studies focused on outcomes in adolescents and young adults, but a slightly poorer survival than in children has been suggested.26
Interpretation of the data in our study is limited by the restricted nature of the information that could be obtained from this population-based (rather than centre-based) source. We did not have access to many important demographic or clinical details, including socioeconomic status, treatment type and compliance, comorbidities, toxicity, treatment centre and biological subtype. However, the strength of our study comes from the unselected nature and large size of the sample.
In conclusion, although these population-based Australian data confirm a clear improvement in survival from haematological malignancies in young people, a survival gap remains for adolescents and young adults compared with children. Greater participation of adolescents and young adults in clinical trials is needed to provide evidence about optimal regimens in these age groups. Formal evaluation of the effect of any changes in health care delivery (such as the adoption of centralised care) on survival in these age groups is also required.
1 Number of new cases of haematological malignancies, by period of diagnosis, Australia
Age (years) |
Period of diagnosis |
|
Sex ratio (male : female) |
||||||||||||
1982–1989 |
1990–1994 |
1995–1999 |
2000–2004 |
Total |
|||||||||||
Acute lymphoblastic leukaemia |
|
|
|
|
|||||||||||
0–14 |
1065 |
735 |
752 |
860 |
3412 |
1.24 |
|||||||||
15–19 |
139 |
108 |
109 |
96 |
452 |
2.12 |
|||||||||
20–29 |
126 |
100 |
93 |
92 |
411 |
1.72 |
|||||||||
Acute myeloid leukaemia |
|||||||||||||||
0–14 |
216 |
154 |
137 |
173 |
680 |
1.19 |
|||||||||
15–19 |
85 |
60 |
60 |
62 |
267 |
1.02 |
|||||||||
20–29 |
187 |
135 |
163 |
158 |
643 |
0.98 |
|||||||||
Non-Hodgkin lymphoma |
|||||||||||||||
0–14 |
251 |
176 |
184 |
182 |
793 |
2.51 |
|||||||||
15–19 |
122 |
88 |
113 |
120 |
443 |
1.95 |
|||||||||
20–29 |
382 |
340 |
360 |
371 |
1453 |
1.75 |
|||||||||
Hodgkin lymphoma |
|
|
|
|
|
||||||||||
0–14 |
128 |
107 |
109 |
126 |
470 |
2.11 |
|||||||||
15–19 |
237 |
178 |
188 |
205 |
808 |
1.00 |
|||||||||
20–29 |
649 |
425 |
487 |
522 |
2083 |
1.11 |
|||||||||
2 Percentage of patients (95% CI) with 5-year survival from haematological malignancies, by period of diagnosis, Australia
Age (years) |
1982–1989 |
1990–1994 |
1995–1999 |
2000–2004 |
|||||||||||
Acute lymphoblastic leukaemia |
|
|
|||||||||||||
0–14 |
73.7 (71.0–76.3) |
75.7 (72.4–78.6) |
82.9 (80.0–85.4) |
87.5 (84.8–89.7) |
|||||||||||
15–19 |
39.6 (31.4–47.6) |
38.0 (28.9–47.0) |
57.8 (48.0–66.4) |
73.6 (62.5–81.8) |
|||||||||||
20–29 |
31.0 (23.1–39.1) |
42.0 (32.3–51.4) |
41.9 (31.8–51.7) |
47.1 (34.5–58.6) |
|||||||||||
Acute myeloid leukaemia |
|
|
|
||||||||||||
0–14 |
38.9 (32.4–45.3) |
49.4 (41.2–57.0) |
51.8 (43.2–59.8) |
69.2 (61.6–75.7) |
|||||||||||
15–19 |
29.4 (20.2–39.3) |
36.7 (24.7–48.7) |
53.3 (40.0–65.0) |
74.2 (61.3–83.3) |
|||||||||||
20–29 |
27.3 (21.1–33.8) |
41.5 (33.1–49.6) |
49.1 (41.2–56.5) |
62.5 (54.1–69.8) |
|||||||||||
Non-Hodgkin lymphoma |
|
|
|
||||||||||||
0–14 |
70.5 (64.5–75.7) |
75.6 (68.5–81.3) |
83.2 (76.9–87.8) |
88.7 (82.6–92.7) |
|||||||||||
15–19 |
63.1 (53.9–71.0) |
71.6 (60.9–79.8) |
75.2 (66.2–82.2) |
83.3 (75.3–88.9) |
|||||||||||
20–29 |
64.4 (59.4–69.0) |
63.2 (57.9–68.1) |
68.9 (63.8–73.4) |
81.1 (76.6–84.8) |
|||||||||||
Hodgkin lymphoma |
|
|
|
||||||||||||
0–14 |
91.4 (85.0–95.2) |
95.3 (89.1–98.0) |
94.5 (88.2–97.5) |
99.2 (94.1–99.9) |
|||||||||||
15–19 |
88.2 (83.4–91.7) |
94.4 (89.8–96.9) |
94.7 (90.3–97.1) |
97.5 (94.0–98.9) |
|||||||||||
20–29 |
86.6 (83.7–89.0) |
92.7 (89.8–94.8) |
95.9 (93.7–97.3) |
95.1 (92.5–96.8) |
|||||||||||
3 Kaplan–Meier estimates of survival probability for patients with haematological malignancies, by age, Australia, 1990–1994 and 2000–2004
4 Recent estimated percentages (95% CI) of 5-year survival for adolescents and young adults versus children in Australia, Europe and the United States
Age (years) |
Region (period of diagnosis) |
||||||||||||||
Australia (2000–2004) |
Europe11 (2000–2002) |
US12,13 (1999–2005)* |
|||||||||||||
Acute lymphoblastic leukaemia |
|
|
|||||||||||||
0–14 |
87.5 (84.8–89.7) |
85.4 (83.7–87.1) |
87.1† |
||||||||||||
15–24 |
66.5 (57.0–74.4) |
49.5 (42.5–56.5) |
54.3 (47.0–61.6) |
||||||||||||
Acute myeloid leukaemia |
|
|
|||||||||||||
0–14 |
69.2 (61.6–75.7) |
66.8 (61.8–71.9) |
60.9† |
||||||||||||
15–24 |
67.5 (58.8–74.8) |
59.1 (50.3–67.9) |
47.2 (39.6–54.8) |
||||||||||||
Non-Hodgkin lymphoma |
|
|
|||||||||||||
0–14 |
88.7 (82.6–92.7) |
82.3 (78.2–86.5) |
84.5† |
||||||||||||
15–24 |
81.6 (76.5–85.8) |
74.4 (69.2–79.5) |
78.4 (74.3–82.5) |
||||||||||||
Hodgkin lymphoma |
|
|
|||||||||||||
0–14 |
99.2 (94.1–99.9) |
95.2 (93.0–97.5) |
94.0† |
||||||||||||
15–24 |
97.1 (94.8–98.4) |
93.1 (91.4–94.9) |
94.8 (93.0–96.6) |
||||||||||||
* Estimates from the US are for 1999–2005 for age 0–14 years and 2001–2005 for age 15–24 years. US estimates are relative survival estimates, which take into account competing causes of death. However, the number of non-cancer deaths among children, adolescents and young adults diagnosed with cancer is small, so that it is valid to compare relative survival estimates from the US with standard Kaplan–Meier estimates from Australia and Europe. † Confidence interval not reported. |
|||||||||||||||
Received 30 July 2009, accepted 11 February 2010
- Ross Pinkerton1
- Rachael-Anne Wills2
- Michael D Coory3
- Christopher J Fraser1
- 1 Queensland Children’s Cancer Centre, Royal Children’s Hospital, Brisbane, QLD.
- 2 Health Statistics Centre, Queensland Health, Brisbane, QLD.
- 3 Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, VIC.
We acknowledge the work of Kun Zhao in extracting data from the ACD and coding the cancer subgroups for us, and we thank the Australian Institute of Health and Welfare for their permission to access and use the data for this study.
None identified.
- 1. Pulte D, Gondos A, Brenner H. Trends in 5- and 10 year survival after diagnosis with childhood hematologic malignancies in the United States, 1990–2004. J Natl Cancer Inst 2008; 100: 1301-1309.
- 2. Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet 2008; 371: 1030-1043.
- 3. Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer 2008; 113: 2575-2596.
- 4. Birch JM, Pang D, Alston RD, et al. Survival from cancer in teenagers and young adults in England, 1979–2003. Br J Cancer 2008; 99: 830-835.
- 5. Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer 2006; 107 (7 Suppl): 1645-1655.
- 6. Ferrari A, Montello M, Budd T, Bleyer A. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer 2008; 50 (5 Suppl): 1101-1104.
- 7. Ramanujachar R, Richards S, Hann I, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXll/E2993) trials. Pediatr Blood Cancer 2007; 48: 254-261.
- 8. Schiffer CA. Differences in outcome in adolescents with acute lymphoblastic leukaemia: a consequence of better regimens? Better doctors? Both? J Clin Oncol 2003; 21: 760-761.
- 9. Möricke A, Zimmermann M, Reiter A, et al. Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. Klin Padiatr 2005; 217: 310-320.
- 10. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer 2005; 103: 1457-1467.
- 11. Gatta G, Zigon G, Capocaccia R, et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer 2009; 45: 992-1005.
- 12. Horner MJ, Ries LAG, Krapcho M, et al; editors. SEER Cancer Statistics Review 1975–2006. Bethesda, Md: National Cancer Institute, 2009.
- 13. Pulte D, Gondos A, Brenner H. Trends in survival after diagnosis with hematologic malignancy in adolescence or young adulthood in the United States, 1981–2005. Cancer 2009; 115: 4973-4979.
- 14. Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood 2008; 112: 1646-1654.
- 15. Burnett A. MRC trials in acute myeloblastic luekaemia [sic]: where have we got to? Leuk Lymphoma 2007; 48: 2289-2290.
- 16. Fagioli F, Zecca M, Locatelli F, et al. Allogeneic stem cell transplantation for children with acute myeloid leukemia in second complete remission. J Pediatr Hematol Oncol 2008; 30: 575-583.
- 17. Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood 2006; 108: 2867-2873.
- 18. Cairo MS, Raetz E, Lim MS, et al. Childhood and adolescent non-Hodgkin lymphoma: new insights in biology and critical challenges for the future. Pediatr Blood Cancer 2005; 45: 753-769.
- 19. Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol 2009; 144: 24-40.
- 20. Pulte D, Gondos A, Brenner H. Ongoing improvement in outcomes for patients diagnosed as having non-Hodgkin lymphoma from the 1990s to the early 21st century. Arch Intern Med 2008; 168: 469-476.
- 21. Hoelzer D, Ludwig WD, Thiel E, et al. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood 1996; 87: 495-508.
- 22. Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7: 379-391.
- 23. Neth O, Seidemann K, Jansen P, et al. Precursor B-cell lymphoblastic lymphoma in childhood and adolescence: clinical features, treatment, and results in trials NHL-BFM 86 and 90. Med Pediatr Oncol 2000; 35: 20-27.
- 24. Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004; 104: 1624-1630.
- 25. Kung FH, Schwartz CL, Ferree CR, et al. POG 8625: a randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with Stages I, IIA, IIIA1 Hodgkin Disease: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol 2006; 28: 362-368.
- 26. Herbertson RA, Evans LS, Hutchinson J, et al. Poor outcome in adolescents with high-risk Hodgkin lymphoma. Int J Oncol 2008; 33: 145-151.





Abstract
Objectives: To examine 5-year survival from haematological malignancies in children, adolescents and young adults in Australia and determine if there has been any improvement in survival for the older age groups compared with children (the age-related “survival gap”).
Design, setting and participants: Population-based study of all Australian children (aged 0–14 years), adolescents (15–19 years) and young adults (20–29 years) diagnosed with acute lymphoblastic leukaemia (ALL), acute myeloid leukaemia (AML), Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) between 1982 and 2004, with follow-up to 2006.
Main outcome measures: 5-year survival from ALL, AML, HL and NHL analysed for four periods of diagnosis (1982–1989, 1990–1994, 1995–1999 and 2000–2004).
Results: During 1982–2004, 13 015 people aged ≤ 29 years were diagnosed with primary leukaemia or lymphoma in Australia. For those with ALL, 5-year survival for adolescents improved from 40% (1982–1989) to 74% (2000–2004); the improvement for young adults was smaller (31% to 47%), and both these groups still had lower survival than children, whose 5-year survival improved from 74% to 88%. There was a larger narrowing of the gap for AML: for cases diagnosed in 2000–2004, 5-year survival was similar for young adults (63%), adolescents (74%) and children (69%). For lymphoma cases diagnosed in 2000–2004, 5-year survival in all age groups was greater than 95% for HL and greater than 81% for NHL, although children fared better than adolescents and young adults.
Conclusions: These Australian population-based data confirm an improvement in survival from haematological malignancies across all three age groups, but an age-related survival gap remains for adolescents and young adults compared with children, especially for young adults with ALL. Greater participation of adolescents and young adults in clinical trials and more detailed data collection are needed to provide evidence about optimal treatment regimens in these age groups.