Although varicella vaccination benefits the community through reducing rates of varicella-zoster virus (VZV) infection in both vaccinated and unvaccinated populations, it may have foreseeable adverse consequences. Exposure to wild-type VZV boosts immunity, which consequently reduces the incidence of herpes zoster (HZ [shingles]).1,2 A possible outcome of the introduction of effective varicella vaccines to Australia (Varilrix, GlaxoSmithKline; and Varivax, Merck Sharp and Dohme) may be an increase in the incidence of HZ in the unvaccinated older population in whom immunity has waned.
Varicella vaccine was licensed in Australia in 1999, became available on private prescription in 2000, and was funded through the National Immunisation Program from November 2005. The program funds varicella vaccination of all children at 18 months of age, as well as school-based programs for vaccination of the “catch-up” cohort of children between 10 and 13 years of age. Before the public program was instituted, vaccine uptake was thought to be low (16%–48% in children under 4 years of age).3,4
In Australia in the financial year 1998–99, just before the introduction of the varicella vaccine, there were 4718 hospitalisations for HZ (mean age of patients, 69 years) and 1991 for varicella (chickenpox) (mean age of patients, 15 years), with respective case-fatality rates of 1% and 0.4%.5 A recent study in Victoria demonstrated a decline in hospitalisation rates for varicella between 2000 and 2007 and an increase in HZ hospitalisation rates from 1998 to 2007.6 It also reported limited community data from the Melbourne Medical Deputising Service that suggested increased HZ presentations in that service between 2000 and 2007.
To build on the observations of this Victorian study, we investigated management rates of both varicella and HZ in Australian general practice for the period April 1998 to March 2009, using a nationally representative sample of general practice consultations available through the BEACH (Bettering the Evaluation and Care of Health) program.7 Our hypothesis was that if the frequency of management of HZ in general practice were shown to be increasing, a pragmatic outcome of this study could be the identification of an age cut-off point for vaccination to prevent subsequent HZ eruptions in older people.
We undertook an analysis of BEACH data from 1998 to 2009 in single data-year blocks (from 1 April to 31 March). Data were collected as described elsewhere.7
Briefly, a national random sample of general practitioners who claimed at least 375 general practice Medicare items of service in the previous quarter are invited to participate in the BEACH study. Each year, about 1000 GPs participate, each providing details of 100 consecutive patient encounters, on structured paper forms, including problems managed and treatments provided. Data collection is evenly distributed throughout 50 weeks of each year (2 weeks’ closure over Christmas). The GP response rate is about 30% each year. The characteristics of participating GPs are compared with those of GPs in the sample frame from which the participants were drawn. Post-stratification age and sex weightings are applied to the encounter data to correct for any under-representations. The encounter data are also weighted according to the activity level of the participating GP (measured by the number of Medicare claims made for GP service items in the 12 months before selection). The resulting age and sex distribution of patients included in the BEACH study who received a GP service covered by a Medicare rebate has been shown to have excellent precision in representing all GP service encounters for which Medicare claims have been made.7
Problems managed at each encounter are classified according to the International Classification of Primary Care, version 2 (ICPC-2).8 Encounters classified as S70: Herpes Zoster, and A72: Chickenpox (Varicella) were included in this study.
For each data year, we recorded the number of encounters for management of varicella and HZ and calculated the management rate per 1000 GP–patient encounters, with 95% confidence intervals, and the mean age of patients. Estimates of the number of times varicella and HZ were managed nationally in general practice were also calculated based on the total number of general practice professional services claimed from Medicare each financial year.9
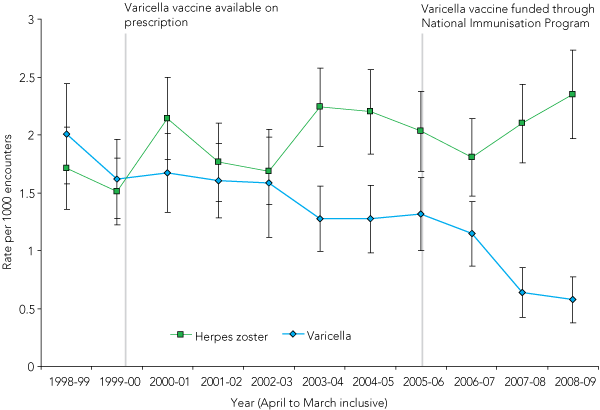
Over the 11 years of BEACH data (April 1998 to March 2009), there were 1 078 671 (weighted) management encounter records with 10 885 GPs. The management rate for HZ was around 1.52 per 1000 encounters at the time the varicella vaccine was made available on private prescription (1999–2000), 2.20 in 2004–2005 and 2.35 in 2008–2009 (Box 1), an increase of 55% since 1999–2000. The confidence intervals around these estimates suggest that this increase was statistically significant. The regression analysis also indicated a significant rise in the HZ management rate over this period, with an average annual increase in HZ management encounters of 0.05 per 1000 encounters (P < 0.01).
The management rate for varicella decreased steadily from 1.61 per 1000 encounters in 1999–2000 to 1.27 per 1000 in 2004–2005, just before acceptance of the vaccine into the National Immunisation Program, and then stayed steady in 2005–2006. From then on, it decreased steadily to 0.58 per 1000 encounters in 2008–2009, significantly less than in all years between 1999–2000 and 2005–2006 (Box 1). The regression analysis also demonstrated a significant decline over the period from 1999–2000 to 2008–2009, with an average annual decrease in varicella management rate of 0.12 per 1000 encounters (P < 0.0001).
Changes in management rates of varicella and HZ per 1000 encounters are shown graphically in Box 2.
Calculation of national estimates from these results suggests that across Australia in 1999–2000 there were about 164 000 encounters at which varicella was managed, decreasing to 65 000 in 2008–2009 (Box 1). In contrast, we estimate that there were 154 000 encounters at which HZ was managed in 1999–2000, and this increased to 266 000 in 2008–2009.
Investigation of the age distribution of the patients managed for HZ in the most recent 3 years demonstrated that 26% were aged less than 50 years, 52% were aged less than 65 years and 71% were aged less than 75 years (Box 3).
In England and Wales, average incidence rates for varicella and HZ were 1291 and 373 per 100 000 person years, respectively, for the years 1991–2000.1 The hospitalisation rates were similar for each disease (4.5 per 100 000 population for varicella versus 4.4 per 100 000 population for HZ), but both inpatient days and the case fatality rate due to HZ were four to six times higher than those due to varicella — a much greater burden of disease. The US Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) reported in 2007 that “multiple studies and surveillance data demonstrate no consistent trends in [HZ] incidence in the United States since implementation of the varicella vaccination program in 1995”,10 whereas there was a significant decrease in varicella over the decade 1992 to 2002.10 Three studies cited in the ACIP report showed no increase in the incidence of HZ.11-13 However, another study that was cited, although finding a similar decrease in varicella in the Massachusetts population, showed a significant near doubling of HZ incidence.14
A single vaccination at the age of 65 years has been proposed as a cost-effective method of dealing with incident HZ in older people.15 The Shingles Prevention Study demonstrated a marked reduction in morbidity from HZ and post-herpetic neuralgia in a large cohort of subjects aged 60 years or older who were vaccinated against HZ compared with placebo.16 The vaccine given to participants (Zostavax, Merck Sharp and Dohme) is a single-dose, live, attenuated VZV vaccine effective in preventing first eruptions of HZ and its associated complication of post-herpetic neuralgia.17 It contains 14 times more plaque forming units than Varivax. Given the burden of disease seen in older people, it may be argued that such a program would be beneficial irrespective of the effects of varicella vaccination of the infant population on HZ incidence in older people.
It would seem pragmatic to introduce an HZ prevention program into general practice for people aged 65 years, as this would correspond to other funded programs for older people, such as influenza and pneumococcal vaccination. Yet, while Zostavax can be administered concomitantly with influenza vaccine, the same cannot be said about the pneumococcal vaccine (Pneumovax23, Merck Sharp and Dohme) as this is associated with a reduced immunological response.17
1 Number, rate and national estimate of encounters for varicella and herpes zoster in general practice, and patient mean age, for each 12 months from April 1998 to March 2009*
- Mark R Nelson1
- Helena C Britt2
- Christopher M Harrison2
- 1 Menzies Research Institute, University of Tasmania, Hobart, TAS.
- 2 Family Medicine Research Centre, School of Public Health, University of Sydney, Sydney, NSW.
During the data collection years reported here, the BEACH program was funded by the Australian Government Department of Health and Ageing, the Australian Institute of Health and Welfare, the National Prescribing Service Ltd, AstraZeneca Pty Ltd (Australia), Roche Products Pty Ltd, Janssen-Cilag Pty Ltd, Merck Sharp and Dohme (Australia) Pty Ltd, Pfizer Australia, Sanofi-Aventis Australia Pty Ltd, Abbott Australasia, Wyeth Australia Pty Ltd, the Australian Government Department of Veterans’ Affairs, and the Office of the Australian Safety and Compensation Council (Department of Employment and Workplace Relations).
Mark Nelson has received an honorarium for preparing educational material for Bristol-Myers Squibb and has participated as a researcher in a trial funded by Merck Sharpe and Dohme.
- 1. Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine 2002; 20: 2500-2507.
- 2. Thomas SL, Wheeler JG, Hall AJ. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet 2002; 360: 678-682.
- 3. Marshall H, Ryan P, Roberton D. Uptake of varicella vaccine — a cross sectional survey of parental attitudes to nationally recommended but unfunded varicella immunisation. Vaccine 2005; 23: 5389–5397.
- 4. Macartney KK, Beutels P, McIntyre P, Burgess MA. Varicella vaccination in Australia. J Paediatr Child Health 2005; 41: 544–552.
- 5. MacIntyre CR, Chu CP, Burgess MA. Use of hospitalization and pharmaceutical prescribing data to compare the prevaccination burden of varicella and herpes zoster in Australia. Epidemiol Infect 2003; 131: 675-682.
- 6. Carville KS, Riddell MA, Kelly HA. A decline in varicella but an uncertain impact on zoster following varicella vaccination in Victoria, Australia. Vaccine 2010; 28: 2532-2538.
- 7. Britt H, Miller GC, Charles J, et al. General practice activity in Australia 2008-09. Canberra: Australian Institute of Health and Welfare, 2009. (AIHW Cat. No. GEP 25.)
- 8. Classification Committee of the World Organization of Family Doctors. ICPC-2: International Classification of Primary Care. 2nd ed. Oxford: Oxford University Press, 1998.
- 9. Medicare Australia. Health statistics, Medicare Benefits Schedule. Canberra: Medicare Australia, 2009. www.medicareaustralia.gov.au/statistics/mbs_item.shtml (accessed Aug 2009).
- 10. Marin M, Güris D, Chaves SS, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56 (RR-4): 1-40. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5604a1.htm (accessed Jun 2010).
- 11. Jumaan AO, Yu O, Jackson LA, et al. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191: 2002-2007.
- 12. Mullooly JP, Riedlinger K, Chun C, et al. Incidence of herpes zoster, 1997-2002. Epidemiol Infect 2005; 133: 245-253.
- 13. Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005; 20: 748-753.
- 14. Yih WK, Brooks DR, Lett SM, et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health 2005; 5: 68. doi: 10.1186/1417-2458-5-68.
- 15. Chapman RS, Cross KW, Fleming DM. The incidence of shingles and its implications for vaccination policy. Vaccine 2003; 21: 2541-2547.
- 16. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352: 2271-2284.
- 17. US National Institutes of Health. ClinicalTrials.gov. Zostavax? administered concomitantly with Pneumovax? 23. http://clinicaltrials.gov/ct2/show/study/NCT0 0535730?sect=X6015 (accessed Jun 2010).





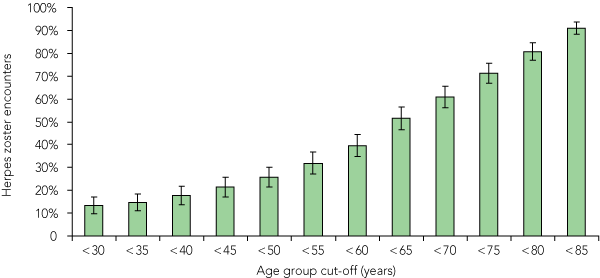
Abstract
Objectives: To assess whether the management rate of herpes zoster (HZ) in Australian general practice has changed since varicella vaccines became available; and to ascertain the mean age of patients attending general practice for HZ management, to assist with planning of vaccination to prevent HZ in older Australians.
Design, setting and participants: Retrospective analysis of data for the period April 1998 to March 2009 on 1 078 671 (weighted) management encounters with consecutive patients of 10 885 general practitioners who participated in the BEACH (Bettering the Evaluation and Care of Health) national cross-sectional survey.
Main outcome measures: Number of encounters for management of HZ (shingles) and of varicella (chickenpox); age of patients presenting for HZ management.
Results: Regression analysis indicated a significant rise in the HZ management rate over the study period, with an average annual increase of 0.05 per 1000 encounters (P < 0.01). The management rate for varicella decreased significantly from 2.01 per 1000 encounters in 1998–1999 to 0.58 per 1000 in 2008–2009. Mean age calculated for each year of the study varied between 10.2 and 15.3 years for patients with varicella, and between 57.5 and 64.1 years for patients with HZ.
Conclusions: There has been a significant rise in the HZ management rate and a decrease in the varicella management rate in Australian general practice over the period 1998–2009. Introduction of vaccination for HZ prevention at age 60 years should be considered, although addition of this vaccination to the existing schedule for vaccination at age 65 years is also likely to be beneficial and may be more pragmatic.