Most guidelines for asthma1,2 and chronic obstructive pulmonary disease (COPD)3,4 stress the importance of spirometry for diagnosis and evaluation of management options. A review of COPD has also recommended that forced expiratory volume in 1 second (FEV1) after administration of a bronchodilator be measured repeatedly over the course of the disease to define the rate of decline in lung function,5 to help focus both treatment decisions and discussions regarding prognosis. This implies that spirometry should be used on a routine basis, to identify patients with a rapid decline in FEV1 and to improve quality of care.
Few long-term studies have evaluated the benefit of regular spirometry in the management of COPD and asthma in general practice. The Detection, Intervention and Monitoring of COPD and Asthma program found that it was possible to detect COPD and asthma at an early stage.6 However, early initiation of inhaled corticosteroids did not demonstrate any effect on the rate of FEV1 decline.7
Victoria has the lowest rate of Medicare claims of any Australian state for spirometry and complex lung function tests, particularly office-based tests,8 despite having a similar prevalence of asthma9 and COPD10 to other states. This presents a unique opportunity to investigate the role of spirometry in improving the management of chronic respiratory diseases. We therefore conducted a randomised controlled trial (RCT) of spirometry with regular medical review as an intervention for managing asthma and COPD in a general practice setting. Our aim was to determine whether this intervention resulted in improvements in quality of life, increased written asthma action plans, or reductions in respiratory symptoms, days lost from usual activities, emergency presentations or hospital admissions, compared with usual care.
Group A: Intervention — 3-monthly spirometry with reports returned to the practice and regular medical review;
Group B: Spirometry only — spirometry at baseline and 12 months, with no report until after completion of the trial; and
Group C: Control — usual medical care (which was likely to vary between practices but did not include regular spirometry).
Eligible patients were those attending participating practices who had been prescribed any inhaled medication in the preceding 6 months, were aged 8–70 years and able to understand English. We excluded patients who were not contactable by telephone, could not speak or read English, were children with infrequent episodic asthma,2 or had other complex medical conditions such as mental illness or cancer. GPs were asked to search their databases for potentially eligible patients and identify the first 50 eligible patients in each of two age groups (8–17 or 18–70 years) to be invited by letter to participate.
Spirometry was performed in the practice by a trained respiratory scientist using a Micro Medical SpiroUSB 36-ML2525 electronic turbine spirometer (Cardinal Health, Basingstoke, UK) before and after administration of salbutamol, in accordance with American Thoracic Society–European Respiratory Society (ATS–ERS) guidelines.11 Measured FEV1 and forced vital capacity were compared with values predicted from the United States National Health and Nutrition Examination Survey.12 The results were interpreted by a respiratory physician or paediatrician following ATS–ERS recommendations.13 A written report was faxed back to the general practice within days, and the patient was encouraged to attend for medical review.
The primary outcome, quality of life, was measured at baseline and then every 3 months with the 36-item Short Form (SF-36) questionnaire, Australian (English) Version 2.14 The SF-36 was scored against US norms to generate Physical Component Summary (PCS) and Mental Component Summary (MCS) scores, as Australian norms were not available for this version.
The secondary outcomes — respiratory symptoms, asthma attacks, written asthma action plans, days lost from usual activities, emergency presentations to the GP or emergency department, and hospital admissions — were assessed with a modified European Community Respiratory Health Survey questionnaire15 (http://www.ecrhs.org), administered at baseline and 12 months.
Allowing for clustering by practice (intracluster correlation, 0.02),16 a 10% dropout rate of practices and patients, and multiple comparisons across three groups (significance level in pairwise comparisons, 0.0167), we required 11 general practices per group to recruit 22 adult patients each. Practices recruited about half this number, so instead of a desired 0.38 SD difference, the study had 80% power (5% two-sided type 1 error in pairwise group comparisons) to detect a difference in mean quality of life of 0.45 SD. These effect sizes corresponded to absolute differences of 4.6 and 5.4, respectively, assuming a similar distribution of PCS scores to another Australian study of asthma patients.17
Hierarchical linear regression models with levels for practice, individuals within practices, and repeated measurements per individual were used to estimate effects of the intervention on SF-36 scores across post-baseline time points. These models included an interaction between randomisation group and time point (3, 6, 9 and 12 months), and adjusted for baseline SF-36 scores, age, sex, diagnosis, smoking history and socioeconomic status. The intervention effects were interpreted as differences between groups in their adjusted mean changes from baseline.18
The secondary analyses compared 12-month outcomes between groups in logistic regression models adjusting for baseline values of the outcomes and age (a variable that exhibited imbalance between groups at baseline). These models were estimated using generalised estimating equations (GEE) with an exchangeable working correlation matrix to reflect clustering at the practice level. GEE provided odds ratio estimates that were population-averaged effects of the interventions in comparison with usual care.19
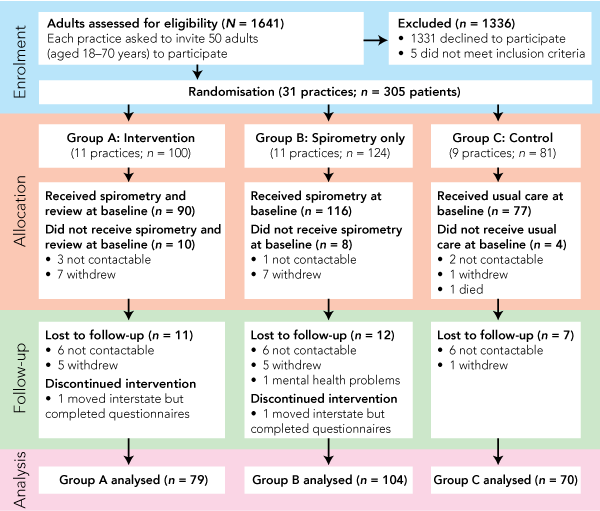
Of 48 practices that agreed to participate, 31 (with 75 full-time and 49 part-time GPs) proceeded to randomisation and recruited 305 patients (Box 1). The other 17 practices (25 full-time and 28 part-time GPs) dropped out before randomisation, due to the principal GP changing his or her mind, and did not recruit any patients.
The flow of participants through the trial is summarised in Box 1. From the 305 adults recruited, 283 baseline questionnaires were returned (90 from intervention practices, 116 from spirometry-only practices and 77 from control practices). Baseline characteristics for the three groups are shown in Box 2. Group B participants were slightly older (P = 0.016), but there were no other significant differences between groups. Doctors’ diagnoses were available for 278 patients: 190 (68%) were considered to have asthma, 36 (13%) had COPD, 40 (14%) had both conditions, and eight (3%) had other conditions such as pulmonary fibrosis, bronchiectasis or undiagnosed cough.
The trial was completed by 253 participants (83%) with a median age of 58 years (range, 18–70 years), of whom 167 (66%) were women. There were 38 current smokers (15%), 102 former smokers (40%), and 113 participants who had never smoked (45%). Losses to follow-up in each group are shown in Box 1. Those who dropped out were slightly younger (median age, 53 years; P = 0.3) and more likely to report chest tightness (76%; P = 0.005), but otherwise were not significantly different from those who completed the trial.
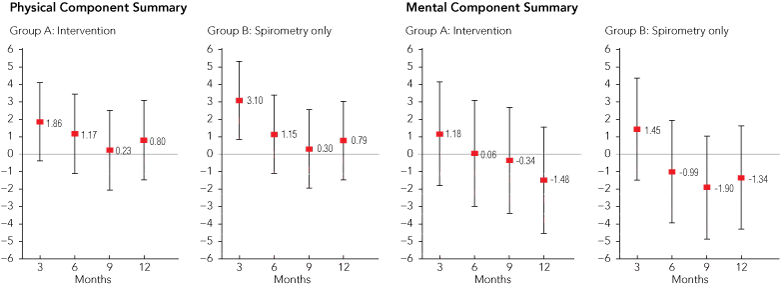
The three groups’ mean scores on the SF-36 subscales, PCS and MCS were well balanced at baseline (Box 3). Longitudinal analyses of PCS and MCS scores are presented graphically in Box 4. There was a small increase in PCS score at 3 months in the spirometry-only group relative to the change seen in controls. However, there was no significant change from baseline in the intervention group compared with controls, nor was there any difference in MCS score change from baseline between either spirometry group and the control group. A post-hoc analysis of PCS and MCS scores stratified by diagnosis did not reveal different effects for asthma compared with COPD with or without asthma (data not shown). Examination of SF-36 subscale scores showed that the brief increase in PCS in the spirometry-only group at 3 months was driven by significant increases in the subscales of role limitations due to physical problems and general health (Box 5).
No significant differences were found between groups in any of the secondary outcomes at 12 months (Box 6). There were no significant reductions in respiratory symptoms or asthma attacks in the previous 12 months, increases in written asthma action plans, or reductions in days lost from usual activities, emergency presentations to the GP or emergency department, or hospital admissions.
The lack of positive findings might reflect the insensitivity of a generic quality-of-life measure such as the SF-36. We chose this well validated and widely used instrument rather than a disease-specific questionnaire because we did not know in advance what proportions of patients would have asthma or COPD. We could instead have used a combination of instruments, such as the Asthma Quality of Life Questionnaire and the St George’s Respiratory Questionnaire, but this would have increased responder burden. Nonetheless, we have previously been able, using the SF-36, to detect effects of respiratory symptoms such as wheezing on the quality of life of young adults.20 Therefore, we do not think that the SF-36 was too insensitive to detect a clinically important change in quality of life, had one occurred.
Practices that participated in the trial were likely to be those most interested in the management of chronic respiratory diseases and might not be representative of Australian general practices. We originally anticipated that half the participants would be children and eventually recruited 305 adults. However, even with 253 adults completing the trial, we still had greater than 80% power to detect a moderate effect of the intervention on quality of life. As shown in Box 4, the 95% confidence intervals around observed differences between groups in change from baseline all excluded + 4.6 units, the planned effect size on the PCS. Nonetheless, power to detect different changes between groups in health service use was limited because of small numbers of events (asthma attacks, presentations or hospital admissions) during the trial. More events would have occurred with a longer duration of follow-up.
The numbers of participants were slightly unbalanced between groups at baseline, because practices randomly assigned to spirometry alone tended to recruit more patients. Losses to follow-up were greater than expected, with 52 patients (17%) not completing the trial. Loss was greater in Groups A and B and could be due to the burden of spirometry, but this is unlikely to explain the negative results. Further analysis based on multiply imputed SF-36 outcomes for people who were lost to follow-up found similar results to those presented in Box 4 (data not shown). There were some protocol violations — 10 patients allocated to Group A and eight in Group B never had spirometry performed. Conversely, at 12-month follow-up, 12 patients in Group C reported having spirometry outside the trial. Nine of these were from three practices, suggesting some heterogeneity of “usual care” between practices. While the intention-to-treat analysis was inherently conservative, it was also the least prone to bias.21
It is quite likely that the trial was adversely affected by co-interventions. During the period that practices were being recruited and patients followed, the National Asthma Council Australia launched a major initiative to promote spirometry to GPs and practice nurses.22 However, these spirometry training workshops were initially directed towards rural areas and only five were conducted in Divisions of General Practice participating in our trial. Furthermore, we were trialling a different model, in which a trained respiratory scientist performed spirometry in the general practice according to ATS–ERS standards, and a specialist report was provided later by a qualified respiratory physician.
Another explanation for the negative results might be a failure by GPs to act on spirometry reports and change patient management. However, around half the patients in Groups A and B had moderate–severe airflow limitation (data not shown), so it is unlikely that patients not considered to require more intensive treatment because they had mild airflow limitation would account for the negative results. Most general practices have well established processes for responding to abnormal pathology and radiology reports and recalling patients for further consultation and referral to specialists as required.23,24 However, this might not extend to the occasional faxed spirometry report. We are currently investigating what occurred in a sample of the consultations by conducting focus groups with patients and in-depth interviews with practice staff.
Regular medical review is only one component of the management of chronic respiratory diseases. For example, pulmonary rehabilitation has been shown to improve quality of life for patients with COPD.25 Spirometry by itself would not be expected to increase referrals for pulmonary rehabilitation. Unfortunately, we did not collect data on patients’ use of outpatient rehabilitation services during this trial.
There are few other studies with which our trial can be directly compared. An RCT in Italian general practices did not find that office-based spirometry improved the accuracy of diagnosis of asthma or COPD.26 However, the trial randomised by patient (leading to frequent protocol violations), did not include administration of a bronchodilator, and did not report patient outcomes. Conversely, a before-and-after study of spirometry in American family medicine practices found that there were changes in the management of 48% of patients with asthma or COPD with completed tests.27 However, due to the lack of a control group, we cannot be confident that these changes resulted from spirometry.
Recently, negative results have been reported from an RCT of spirometry conducted in South Australian and Tasmanian general practices.28 The trial did not find any improvement in quality of life, days off work or school, asthma exacerbations, or daytime or nocturnal symptoms in either adults or children with asthma whose management was guided by spirometry. However, the trial’s intervention model was different from ours — GPs and practice nurses were given training in spirometry so they could perform it themselves in their practices. The standards and interpretation of spirometry were also less certain compared with our study.
2 Baseline demographics, smoking status, diagnosis, and symptoms and health care use during the preceding 12 months, by group*
3 Mean (SD) scores at baseline on 36-item Short Form (SF-36) subscales, Physical Component Summary and Mental Component Summary, by group
4 Mean changes from baseline to 3, 6, 9 and 12 months on 36-item Short Form (SF-36) Physical and Mental Component Summary scores*
 | |||||||||||||||
5 Longitudinal analysis of 36-item Short Form (SF-36) subscales*
* Adjusted for baseline score, age, sex, diagnosis, smoking history and socioeconomic status. |
|||||||||||||||
- Michael J Abramson1
- Rosa L Schattner1
- Nabil D Sulaiman2
- Kate E Birch1
- Pam P Simpson1
- Eleonora A Del Colle3
- Rosalie A Aroni4
- Rory Wolfe1
- Francis C K Thien5
- 1 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
- 2 Department of General Practice, University of Melbourne, Melbourne, VIC.
- 3 Pulmetrics Pty Ltd, Melbourne, VIC.
- 4 Department of Health Social Science, Monash University, Melbourne, VIC.
- 5 Eastern Health, Box Hill Hospital, Melbourne, VIC.
The trial was funded by the National Health and Medical Research Council (NHMRC). We thank Anne Adikari, Lorien Barrie, Bernadette Flanagan, Joan Green and Tom Lanigan for data collection and entry. Spirometry was reported by Amanda Griffiths, Celia Lanteri, John Massie, Jennifer Perrett and Helen Whitford. The advisory board comprised Anne Bourke, Geoff Broomhall, Max de Clifford, Fiona Duffy, David Hayne, Amjad Hussain, Gary Irving, John Massie, Judi Wicking and Doris Young. We also thank all participating Divisions of General Practice, GPs, practice staff and patients.
Eleonora Del Colle is the Director of Pulmetrics, the company that performed the spirometry for the study, and is one of the principal authors and a presenter of the National Asthma Council Australia’s Spirometry Training Course for GPs, for which she received an honorarium.
- 1. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Bethesda, Md: GINA, 2009. http://www.ginasthma.com/Guidelineitem.asp??l1=2&l2=1&intId=1561 (accessed Jun 2010).
- 2. National Asthma Council Australia. Asthma management handbook 2006. Melbourne: NAC, 2006.
- 3. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2009). GOLD, 2009. http://www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=2003 (accessed Dec 2009).
- 4. McKenzie DK, Abramson M, Crockett AJ, et al; The Australian Lung Foundation. The COPD-X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2009. http://www.copdx.org.au/guidelines/index.asp (accessed Jun 2010, link no longer available).
- 5. Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2689-2697.
- 6. van den Boom G, van Schayck CP, van Möllen MP, et al. Active detection of chronic obstructive pulmonary disease and asthma in the general population. Results and economic consequences of the DIMCA program. Am J Respir Crit Care Med 1998; 158: 1730-1738.
- 7. van Grunsven P, Schermer T, Akkermans R, et al. Short- and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir Med 2003; 97: 1303-1312.
- 8. Australian Centre for Asthma Monitoring. Asthma in Australia 2005. Canberra: AIHW, 2005. (AIHW Cat. No. ACM 6.) http://www.asthmamonitoring.org/asthma_aust05_html/Index.htm (accessed Dec 2009).
- 9. Robertson CF, Dalton MF, Peat JK, et al. Asthma and other atopic diseases in Australian children. Australian arm of the International Study of Asthma and Allergy in Childhood. Med J Aust 1998; 168: 434-438. <MJA full text>
- 10. Abramson MJ. Respiratory symptoms and lung function in older people with asthma or chronic obstructive pulmonary disease. Med J Aust 2005; 183 (1 Suppl): S23-S25. <MJA full text>
- 11. Miller MR, Hankinson J, Brusasco V, et al; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005; 26: 319-338.
- 12. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999; 159: 179-187.
- 13. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948-968.
- 14. Ware JE, Kosinski M, Gandek B. SF-36 Health Survey manual and interpretation guide. Lincoln, RI: QualityMetric Inc, 2000.
- 15. Burney PGJ, Luczynska C, Chinn S, Jarvis D. The European Community Respiratory Health Survey. Eur Respir J 1994; 7: 954-960.
- 16. Lowe A, Campbell D, Walker P, et al. Clustering effects of chronic obstructive pulmonary disease specific quality of life in hospitalized patients. Respirology 2003; 8: 339-343.
- 17. Adams RJ, Wilson D, Smith BJ, Ruffin RE. Impact of coping and socioeconomic factors on quality of life in adults with asthma. Respirology 2004; 9: 87-95.
- 18. Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ 2001; 323: 1123-1124.
- 19. Hu FB, Goldberg J, Hedeker D, et al. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. Am J Epidemiol 1998; 147: 694-703.
- 20. Matheson M, Raven J, Woods RK, et al. Wheeze not current asthma affects quality of life in young adults with asthma. Thorax 2002; 57: 165-167.
- 21. Piantadosi S. Clinical trials: a methodologic perspective. 2nd ed. Hoboken, NJ: Wiley-Interscience, 2005.
- 22. National Asthma Council Australia. Spirometry training course. http://www.nationalasthma.org.au/content/view/250/630 (accessed Jun 2010).
- 23. Bird S. A GP’s duty to follow up test results. Aust Fam Physician 2003; 32: 45-46.
- 24. Makeham MAB, Saltman DC, Kidd MR. Lessons from the TAPS study — recall and reminder systems. Aust Fam Physician 2008; 37: 923-924.
- 25. Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; (4): CD003793.
- 26. Lusuardi M, De Benedetto F, Paggiaro P, et al. A randomized controlled trial on office spirometry in asthma and COPD in standard general practice: data from spirometry in asthma and COPD: a comparative evaluation Italian study. Chest 2006; 129: 844-852.
- 27. Yawn BP, Enright PL, Lemanske RF Jr, et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest 2007; 132: 1162-1168.
- 28. Beilby J, Nelson MR, Holton C, et al. Spirometry training for GPs and practice nurses: is it feasible and beneficial to asthma patients? In: 2008 General Practice and Primary Health Care Research Conference: Program and Abstracts; 2008 Jun 4; Hobart, Tasmania. Adelaide: Primary Health Care Research and Information Service; 2008.





Abstract
Objective: To determine whether spirometry with regular medical review improves the quality of life or other health outcomes among patients with asthma or chronic obstructive pulmonary disease (COPD) managed in general practice.
Design, setting and participants: Cluster randomised controlled trial conducted in 31 general practices in Melbourne during 2007–2008. Practices recruited 305 adult patients who had been prescribed inhaled medication in the preceding 6 months.
Intervention: Practices were randomly assigned to one of three groups: Group A patients received 3-monthly spirometry performed by a respiratory scientist with results returned to the practice and regular medical review; Group B patients received spirometry only before and after the trial; and Group C patients received usual care.
Main outcome measures: Quality of life, assessed with the 36-item Short Form (SF-36) Australian (English) Version 2 questionnaire at baseline and 3, 6, 9 and 12 months. Secondary outcomes were assessed with the European Community Respiratory Health Survey at baseline and 12 months.
Results: The trial was completed by 253 participants: 79 in Group A, 104 in Group B, and 70 in Group C. Median age was 58 years (range, 18–70 years), and 167 participants (66%) were women. There were no significant changes in SF-36 Physical and Mental Component Summary scores from baseline to 12 months, or significant differences between groups on either scale or any subscale of the SF-36. There were also no significant differences in respiratory symptoms, asthma attacks, written asthma action plans, days lost from usual activities or health care utilisation.
Conclusion: Three-monthly spirometry and regular medical reviews by general practitioners are not associated with any significant improvement in quality of life or other health outcomes for patients with asthma and/or COPD.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12606000378527.