The Pharmaceutical Benefits Scheme (PBS) subsidises three-quarters of the cost of prescription drugs consumed in Australia.1 PBS expenditure has risen rapidly since the early 1990s and is currently about $7 billion per year.2 Observing these trends, the Treasury argued, in its first intergenerational report, that spending on pharmaceuticals is likely to be the largest single contributor to the increasing cost of our ageing population.3
Historically, pharmaceutical costs in Australia were lower than in many comparable countries. In 1996, the Industry Commission4 found that the average prices of the 20 highest cost drugs in Australia were 17% lower than in England. However, there have since been significant changes to the composition of drugs listed under the Schedule of Pharmaceutical Benefits. In particular, the proportion of drugs that are not under patent has risen,5 and is predicted to increase further in the next few years.6
A patent grants the holder a time-limited monopoly right to produce a drug. During the patent period, the price of a drug should reflect the cost of manufacture plus a return on the intellectual property associated with its development, and thereby provide incentives for research and development.7 In the case of generic drugs, the monopoly right has expired and other companies can enter the market; hence price no longer needs to provide a return on the development cost.
The pricing of pharmaceuticals in Australia has also undergone significant changes. In the past, reference pricing was used — reimbursements to patients were set to the level of the lowest-priced drug in a therapeutically equivalent group.8 When a generic equivalent for a drug became available at a lower price, the level of reimbursement was reset to that lower price for all drugs in the therapeutic class. However, reference pricing has not been applied to some statins (hydroxymethylglutaryl-CoA [HMG-CoA] reductase inhibitors) owing to variation in efficacy. For example, the Pharmaceutical Benefits Advisory Committee considered atorvastatin to be more effective than simvastatin when the first generic brands of simvastatin were introduced, so reimbursement for atorvastatin was higher.9
In 2007, the PBS was divided into two separate formularies:10 F1 is intended for single-brand patented medications (this currently includes atorvastatin [whose patent expires in 201211] and rosuvastatin); and F2 is for medications whose patent has expired and for which generic medications can become available (this includes simvastatin and pravastatin).
The degree to which generic statins affect pharmaceutical expenditure is therefore determined by the proportion of statins that are subject to price reductions, which depends on three factors. First, price reductions of statins listed on F2 will not affect the prices of those that remain on F1. Second, if new statins (eg, pitavastatin12) are introduced and listed on F1, they are not affected by generic pricing policies. Third, patent holders may use evergreening strategies to seek extension of the period in which they are able to charge a price premium for their product.13 In Australia, this can occur when a statin is combined with another therapy in a fixed combination. Under the National Health Act, combination products that can be demonstrated to significantly increase compliance may be exempt from future price reductions.14
Prices for generic statins in Australia and England from January 2002 to October 2009 were compared by focusing on underlying wholesale prices, as was done in previous international comparisons.4 Price mark-ups and service fees relating to prescriptions in Australia and England that are not directly comparable were excluded from our analysis.
The Schedule of Pharmaceutical Benefits, which contains the dispensed price of all government-subsidised pharmaceuticals in Australia, was used to obtain statin prices for Australia. These were used to calculate prices by deducting the wholesale mark-up (7.5%), the pharmacist mark-up (10%–15%) and the Consumer Price Index adjusted dispensing fee.15 Comparable data for England were obtained from information on net ingredient costs contained in yearly prescription cost analysis publications.16,17
We also calculated the proportion of total prescriptions that were for patented formulations for the 5 years after patent expiry. As there is often a period of adjustment, we made these comparisons relative to year of patent expiry in England and Australia. For example, in the case of simvastatin, we compared price for 2003 in England with price for 2005 in Australia. English drug costs17 were converted into Australian dollars using annual exchange rates.
The combined cost of all statin therapies to the Australian government (PBS expenditure) and Australian consumers between January 2009 and December 2019 was projected under various scenarios regarding pricing (including the proportion of statins that are subject to price reductions) and future patterns of prescribing. The projections were based on recent trends in price changes for generic statins in Australia, and on both Australian and English prices. The pricing assumptions took into account a recent budget change that placed rosuvastatin and atorvastatin into a single therapeutic group.18 Full documentation of the assumptions underlying these projections is provided at <http://www.health.usyd.edu.au/heconomics/resources/>. For each scenario, we report the net present value of total expenditure to both the government and consumers discounted at a 5% rate per year, which has the effect of reducing expenditures occurring further into the future.
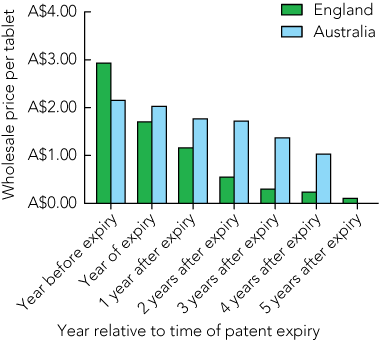
Box 1 shows wholesale prices of the most commonly prescribed dose of simvastatin (40 mg) in Australia and England. While under patent, 40 mg simvastatin originally cost just over $2.00 per tablet in Australia. After patent expiry, the price decreased by an average of 15% per year to around $1.00 per tablet 4 years after patent expiry. In England, the price before patent expiry was higher, but subsequent price decrements occurred at more than twice the rate than in Australia. So, 4 years after patent expiry, the Australian price was four times higher than the English price. The price in England has continued to decline: in 2008 (5 years after expiry of the patent) the cost was $0.11 per tablet.
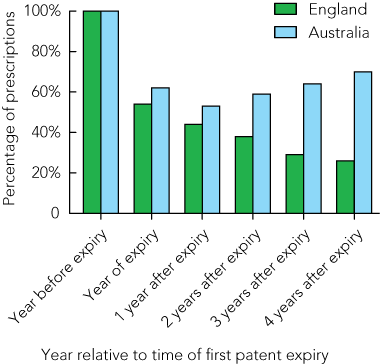
Box 2 shows the proportion of total statin prescriptions accounted for by patented formulations in Australia and England. Since the expiry of the patent for simvastatin in England in 2003, the proportion of patented statin medications prescribed has steadily declined to 26%. In Australia, the opposite occurred, and patented statins (ie, atorvastatin and rosuvastatin) accounted for 70% of statin prescriptions by 4 years after the first statin patent expiry.
Box 3 shows the net present value of the projected expenditure on statins from January 2009 to December 2019 using different scenarios, including government expenditure (subsidies for PBS medications) and consumer expenditure (from co-payments), based on different assumptions regarding the proportion of prescriptions subject to generic price reductions. For example, if 50% of statins were subject to generic price reductions during this period, the projected total expenditure (government plus consumer) on statins would be about $10.4 billion if we continued paying Australian prices that reflect past price decreases. But if price decreases were accelerated to match those of England, expenditure could be reduced to $8.68 billion — a saving of $1.72 billion.
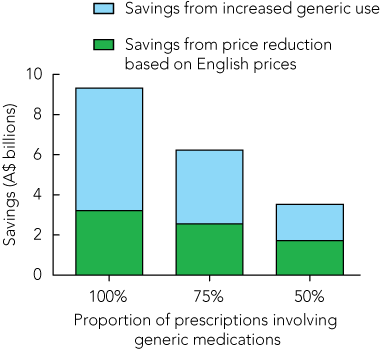
Even greater reductions in expenditure would be possible in Australia during this period if a higher proportion of statins were subject to generic price reductions. Box 4 shows the potential reductions in expenditure that could be achieved, by increasing the use of generics and using English prices, relative to the current situation where about 25% of statin prescriptions are for non-patented formulations. For example, if all prescriptions were for generic statin medications and the lower English prices were applied, total expenditure could be reduced to $2.9 billion — a saving of $9.31 billion ($6.11 billion due to generic substitution). Even increasing generic use to 50% and adopting English prices would decrease expenditure by $3.53 billion.
From January 2005 to October 2009, Australians paid $900 million more for statins than they would have if the prices were equivalent to those in England. Based on recent trends in generic statin pricing, up to $3.21 billion extra will be spent on statins from January 2009 to December 2019 if statin prescriptions are subject to price reductions based on past Australian pricing trends. By increasing the proportion of generic prescriptions to 100% and paying English prices, PBS expenditure on statins could be reduced by up to $9.31 billion. These findings extend the observations of several recent reports which show that Australian prices for generics are higher than in other countries, including New Zealand8 and the United States,19 at a point in time.
Although we focused on comparisons with England, the costs of statins are also significantly lower elsewhere. The wholesale price per 40 mg tablet of simvastatin in Australia is about $1.00,20 whereas it is 5 cents in New Zealand,21 and the United States retail chain Walmart sells pravastatin for 12 cents per tablet.22 If these prices had been used in our analysis, the excess costs would have been even greater.
Furthermore, we have confined our analysis to statins, which account for 16% of PBS funds.23 Additional savings may be possible by reducing the prices of generics in other therapeutic classes over the next few years. For example, further savings could be achieved when the patents on angiotensin II receptor blockers expire.24
The most recent changes to generic pricing arrangements in Australia involve the use of weighted average price calculated using information from manufacturers who disclose to the government the prices charged to pharmacies for their products. While this is similar to the generic pricing mechanism in England,25 it is unclear whether this will bring Australian generic prices into line with those in other countries because price reductions depend on significant competition in the generic market.26 Given the large and growing price disparities between Australia and many other countries, the merits of other approaches that provide incentives to discount prices should be explored.19 Alternatively, a system of competitive tendering for the supply of major generic products such as statins27 similar to the system in New Zealand would be worth considering, as would the reinstatement of reference pricing in which all statins are treated as a single therapeutic class.
We have shown that the use of generic statins has grown rapidly in England, where prescribing of lower-cost medications is recommended on initiation of statin therapy.28 But the reverse has occured in Australia, where patented statin formulations have gained increasing market share. In future, the use of statins as part of combination therapies may also lead to a significant proportion of medications being exempt from price reductions.
The key question is whether the health benefits resulting from using statins under patent or combination therapies justify the substantially higher subsidies from the PBS. Such a question could be answered by examining the incremental cost-effectiveness of these drug formulations over generic alternatives (ie, an evaluation that takes into account the additional benefits conferred in relation to the additional cost). While this has been examined in the other countries,29,30 there has been little consideration of this question in Australia.
With an ageing population and the availability of new therapeutic technologies, the upward trend in pharmaceutical expenditure in Australia is likely to continue. Finding ways to finance this increased expenditure is becoming difficult, especially given the recent deterioration in federal government revenue, which has resulted in a sizeable budget deficit.31 In these circumstances, it is likely that savings will need to be found in the health sector to offset new health expenditure. One of the most effective strategies for limiting growth in PBS expenditure is to pay the lowest possible price for generic medications while maintaining quality and ensuring continuity of supply.
1 Price of 40 mg simvastatin in Australia compared with England, by year relative to patent expiry*
 |
|
* Price data for Australia were unavailable for 5 years after expiry. |
Received 1 September 2009, accepted 27 January 2010
- Philip M Clarke1
- Edmund M Fitzgerald2
- School of Public Health, University of Sydney, Sydney, NSW.
We thank Professor Stephen Leeder for his advice on aspects of this study. Philip Clarke was supported by an NHMRC Career Development Award (571122).
None identified.
- 1. Sweeny K. Key aspects of the Australian Pharmaceutical Benefits Scheme. Working Paper No. 35. Pharmaceutical Industry Project Working Paper Series. Melbourne: Victoria University of Technology, 2007.
- 2. Australian Institute of Health and Welfare. Australia’s health 2008. Canberra: AIHW, 2008. (AIHW Cat. No. AUS 99.)
- 3. Costello P. Intergenerational report 2002–03. Budget Paper No. 5. Canberra: Commonwealth of Australia, 2002.
- 4. Industry Commission. The pharmaceutical industry. Volume 2: appendices. Report No. 51. Melbourne: Australian Government Publishing Service, 1996.
- 5. Lofgren H. Reshaping Australian drug policy: the dilemmas of generic medicines policy. Aust New Zealand Health Policy 2007; 4: 11.
- 6. Beecroft G. Generic drug policy in Australia: a community pharmacy perspective. Aust New Zealand Health Policy 2007; 4: 7.
- 7. Zweifel P, Breyer F. Health economics. New York: Oxford University Press, 1997.
- 8. Searles A, Jefferys S, Doran E, Henry DA. Reference pricing, generic drugs and proposed changes to the Pharmaceutical Benefits Scheme. Med J Aust 2007; 187: 236-239. <MJA full text>
- 9. National Prescribing Service. Atorvastatin (Lipitor) for the management of lipid disorders. NPS RADAR fact sheet, December 2005. http://www.nps.org.au/__data/assets/pdf_file/0007/14686/atorvastatin.pdf (accessed Jan 2010).
- 10. Australian Government Department of Health and Ageing. Fact sheet: Pharmaceutical Benefits Scheme (PBS) reform. http://www.health.gov.au/internet/main/publishing.nsf/Content/pbs_reform_02feb07.htm (accessed Jan 2009).
- 11. Freehills. Atorvastatin in Australia: the latest from the Federal Court. http://www.freehills.com/3200.aspx (accessed Aug 2009).
- 12. Saito Y, Yamada N, Teramoto T, et al. A randomized, double-blind trial comparing the efficacy and safety of pitavastatin versus pravastatin in patients with primary hypercholesterolemia. Atherosclerosis 2002; 162: 373-379.
- 13. Faunce T, Vines T, Gibbons H. New forms of evergreening in Australia: misleading advertising, enantiomers and data exclusivity: Apotex v Servier and Alphapharm v Lundbeck. J Law Med 2008; 16: 220-232.
- 14. Australian Government Department of Health and Ageing. Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (Version 4.3). Canberra: Commonwealth of Australia, 2008.
- 15. Medicare Australia. Pricing of PBS medicine. http://www.medicareaustralia.gov.au/provider/pbs/pharmacists/pricing.jsp (accessed Aug 2009).
- 16. National Health Service Prescription Services. Prescribing information terms. http://www.ppa.nhs.uk//ppa/pi_pmd_report_guidance/prescribing_info_term_files/n.htm (accessed Aug 2009).
- 17. National Health Service Information Centre. Prescription cost analysis 2008. http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescriptions/prescription-cost-analysis-2008 (accessed Apr 2009).
- 18. Australian Government. Part 2: expense measures. Health and ageing. In: Budget measures. Budget Paper No. 2. Canberra: Commonwealth of Australia, 2009. http://www.aph.gov.au/budget/2009-10/content/bp2/html/bp2_expense-16.htm (accessed Jul 2009).
- 19. Bulfone L. High prices for generics in Australia — more competition might help. Aust Health Rev 2009; 33: 200-214.
- 20. Department of Health and Ageing. Schedule of Pharmaceutical Benefits 1 January 2009. Canberra: Commonwealth of Australia, 2009.
- 21. Pharmaceutical Management Agency of New Zealand. Tender results. 30 January 2009. http://www.pharmac.govt.nz/2009/01/30/2009-01-30%20Tender%20Notification.pdf (accessed Aug 2009).
- 22. Walmart. Retail prescription program drug list. Revised 3/3/10. http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf (accessed Apr 2010).
- 23. Stocks N, Ryan P, Allan J, et al. Gender, socioeconomic status, need or access? Differences in statin prescribing across urban, rural and remote Australia. Aust J Rural Health 2009; 17: 92-96.
- 24. Emerton D. Patent expiries in the US statin market: generics to slash market size by 80 per cent over the next ten years. J Generic Medicines 2006; 4: 73-78.
- 25. Department of Health. New long-term arrangements for reimbursement of generic medicines: Scheme M. London: DoH, 2005. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4114256.pdf (accessed Jan 2010).
- 26. de Boer R. PBS reform — a missed opportunity? Aust Health Rev 2009; 33: 176-185.
- 27. Faunce TA, Lofgren H, Harvey K, Johnson K. Tendering for low cost generics in Australia. Health Issues 2006; 87: 26-29.
- 28. National Institute for Health and Clinical Excellence. Statins for the prevention of cardiovascular events. Technology Appraisal 94. London: NICE, 2006.
- 29. Moon JC, Bogle RG. Switching statins. BMJ 2006; 332: 1344-1345.
- 30. Gumbs PD, Verschuren WM, Souverein PC, et al. Society already achieves economic benefits from generic substitution but fails to do the same for therapeutic substitution. Br J Clin Pharmacol 2007; 64: 680-685.
- 31. Australian Government. Budget strategy and outlook. Budget Paper No. 1. Canberra: Commonwealth of Australia, 2009. http://www.budget.gov.au/2009-10/content/overview/html/index.htm (accessed Aug 2009).





Abstract
Objective: To compare changes in the costs of statins following patent expiry in Australia and England, and to estimate projected savings for Australia based on the government and consumers paying prices equivalent to those in England and increased use of generics.
Design: Review of administrative data and predictive models based on recent trends.
Setting: Administrative price and quantity data for the Pharmaceutical Benefits Scheme between January 2002 and October 2009, and comparable information from England.
Main outcome measures: Total government and consumer expenditure on statins whose patent has expired, and projected expenditure on all statins from January 2009 to December 2019 under various scenarios regarding pricing and prescribing trends.
Results: From January 2005 to October 2009, the cumulative loss to the Australian community from paying more than the English price for generic statins was more than $900 million. Expenditure could have been reduced by a further $1087 million if Australia had increased the proportion of generic medications prescribed to match trends in England. Future savings depend on the proportion of statin prescriptions that are subject to lower generic pricing. From January 2009 to December 2019, potential savings from paying English prices could be as high as $3.21 billion, and savings of up to $9.31 billion could be made by paying English prices and using generic statins only.
Conclusion: The current arrangement for pricing statins places a considerable burden on the Australian community. Alternative pricing arrangements that provide incentives to lower statin prices and increase the proportion of generic prescriptions could be highly advantageous.