Many people with type 1 diabetes mellitus (T1DM) struggle with the everyday challenge of attempting to achieve near-normal glycaemia while avoiding hypoglycaemia. The Dose Adjustment for Normal Eating (DAFNE) program of structured education for people with T1DM,1 developed in the United Kingdom by adapting a German diabetes teaching and treatment program,2 offers one potential approach to managing this problem. Reported benefits of DAFNE training include improved overall glycaemic control, reduced hypoglycaemia and improved quality of life.1,2
Participants agreed to the use of their de-identified collective data for quality assurance purposes, but no formal consent form was used. The data collection and reporting process was reviewed by the Mater Health Services Human Research Ethics Committee and the study was exempted as a quality assurance activity, consistent with National Health and Medical Research Council guidelines.3
Data items included participants’ demographics, anthropometric data, history of diabetes and its complications, biochemical variables and scores from two measures of quality of life — the Hospital Anxiety and Depression Scale (HADS)4 and the Problem Areas in Diabetes (PAID) Scale5 — which have been validated in people with diabetes. The HADS is used to assess both anxiety and depressive symptoms. From a possible total score of 21 over both domains, scores < 7 are considered normal, 8–10 suggestive of anxiety or depression, and ≥ 11 indicative of a probable mood disorder. The PAID score covers a range of emotional states frequently reported in diabetes. These data were collected within 1 month of beginning and 1 year after participation in the DAFNE program. Biochemical variables were analysed using routine methods at each local DAFNE centre, without standardisation across centres.
Baseline data for 446 DAFNE course participants were recorded in the system. Of these, 272 had completed DAFNE training at least 1 year before the locking of the database and were eligible for inclusion. One-hundred and fifty-five people had at least some recorded data 1 year after training, and 145 (53%) had HbA1c levels recorded both before and after DAFNE training. These 145 people form the cohort for this report. The number of participants with data recorded varies for different characteristics. Baseline characteristics of the people included and excluded from this study are presented in Box 1. Only the frequency of diabetic peripheral neuropathy varied significantly between these two groups. Nearly all of our final cohort (97%) were white. Participants’ mean body mass index (BMI) was in the overweight range. Twenty-eight per cent had known complications of diabetes.
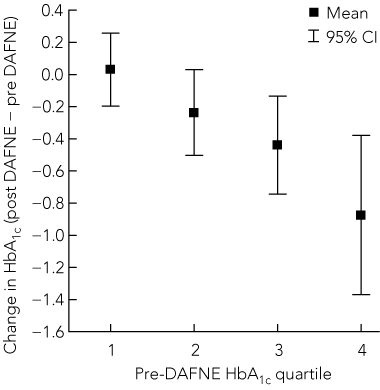
Mean HbA1c levels for the whole cohort fell from 8.2% to 7.8% (95% CI for change, − 0.5% to − 0.2%; P < 0.0001), and the change in HbA1c varied according to baseline glycaemic control, being greater for participants with HbA1c levels in the highest quartile before DAFNE training (Box 2).
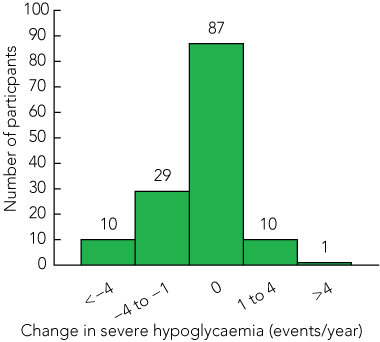
Thirty-four per cent of participants (48/143) reported severe hypoglycaemic events (defined as an episode requiring the assistance of a third party) in the year before they undertook DAFNE training (Box 1), with 13 people reporting more than four events. Overall, severe hypoglycaemic events were less frequent in the 12 months after DAFNE training (P < 0.0001). The distribution of severe hypoglycaemic events was markedly skewed at baseline, with the remaining 66% of participants (95/143) reporting no events in the previous year.
The change in number of severe hypoglycaemic events per year confirms an overall improvement after DAFNE training (Box 3). Eight per cent (11/137) of participants reported an increase in frequency of severe hypoglycaemia in the year after DAFNE training. This group had a longer duration of diabetes, but did not differ in any of the other baseline characteristics. Twenty-eight percent (39/137) reported a decrease in frequency of severe hypoglycaemia. Eighty-three of 87 people who reported no change in hypoglycaemia frequency had not experienced severe hypoglycaemia in the year before DAFNE training.
Participants’ mean weight had fallen by 0.9 kg 12 months after DAFNE participation (95% CI for change, − 1.6 to − 0.2 kg; P = 0.012) (Box 4).
Assessment using the PAID and HADS questionnaires showed that participants’ quality of life had improved 1 year after DAFNE training. The median PAID score before DAFNE training was 25, typical for an unselected outpatient population.5 This decreased to 16.25 (consistent with improved quality of life) 1 year after DAFNE training, corresponding to a small-to-moderate effect size (Box 4). Before training, participants’ PAID scores were highest for questions relating to hypoglycaemia, the future risk of complications, and guilt and anxiety for getting “off track” with diabetes management. The greatest reductions after training were seen in these areas and in concerns regarding food and eating.
The median scores for both HADS questionnaires showed small to moderate reductions after DAFNE training (Box 4). At the 12-month follow-up, a greater proportion of participants showed scores in the normal and mid ranges, with fewer having scores that are likely to indicate a mood disorder.
Our audit of clinical outcomes suggests that the DAFNE program benefits people with T1DM in Australia. The results are broadly consistent with those reported in the UK DAFNE randomised controlled trial1 and in a large-scale German audit series.6 The UK trial reported a larger (about 1%) mean reduction in HbA1c levels, but it had recruited patients with poor glycaemic control and a mean HbA1c level of 9.3% at baseline. Consistent with our findings, the German audit described differences in HbA1c reduction according to baseline HbA1c quartile.
The landmark Diabetes Control and Complications Trial (DCCT)7 offered the first clear evidence that improved glycaemic control in T1DM improves clinical (in particular, microvascular) outcomes, but both the DCCT8 and a subsequent meta-analysis9 suggested that HbA1c reduction must inevitably be accompanied by an increase in severe hypoglycaemia. This, in addition to the weight gain associated with intensive therapy,7 has served to discourage many people with diabetes, and their treating doctors, from attempting more intensive glycaemic control.
However, both our data and the UK and German reports1,6 demonstrate that it is possible to lower HbA1c levels without these unwanted effects using the DAFNE structured education program. We suggest that these benefits may derive from the very detailed 5-day program of patient education that comprises the DAFNE course and from the less stringent glycaemic targets used in DAFNE. While the DCCT program was largely doctor-, dietitian- and nurse-driven, with prescription of dietary regimens, and insulin doses adjusted by clinicians, DAFNE promotes self-management with dietary flexibility and self-titration of insulin doses.
Type 1 diabetes is a profoundly vexatious chronic disease for many people, as it involves complex juggling of insulin doses according to food intake, glucose concentrations, exercise and other, often apparently random, factors on an unremitting daily basis. This clearly can impair quality of life. Although quality of life was not markedly abnormal in our cohort at baseline, all measures did improve after participation in the DAFNE course. The reasons for this may include reduced hypoglycaemia,10 improved self-management skills and confidence, and building a relationship with the health professional team, as well as the supportive environment and sharing of experiences of the “diabetic life” which, anecdotally, are always part of the DAFNE group dynamic.
People with T1DM participating in DAFNE may not be representative of the population of people with T1DM as a whole. However, the mean baseline HbA1c level and BMI for our cohort were very similar to reported values for the cohort of T1DM patients who participated in the 2006 Australian National Diabetes Information Audit and Benchmarking survey of patients attending specialist diabetes clinics in Australia (HbA1c, 8.1% ± 1.6%; BMI, 26.2 ± 4.5 kg/m2).11 The distribution of HADS scores in our cohort at baseline was also similar to that reported in a UK cohort of adults with T1DM.4 The reasons why some participants did not attend for data collection 1 year after DAFNE training are not known, but these participants do not appear to differ in terms of baseline clinical characteristics from those who did attend. This audit reports outcomes on a pre- and post-intervention basis, with no control group to assess changes over time without the intervention. However, such data are available from the UK DAFNE study1 and our results are consistent with other published reports.2,6
Structured patient education has been endorsed as a routine part of diabetes management by the National Institute for Clinical Excellence in the UK,12 with DAFNE being recognised as one program suitable for people with T1DM. Cost modelling in the UK has suggested that DAFNE is cost-saving, due to reduced diabetic complications, rather than just cost-effective.13 Other structured education courses, such as the Empowerment program developed and conducted in Newcastle, Australia,14 and flexible insulin therapy teaching courses in Basel, Switzerland,15 have also shown benefits, including reduced hypoglycaemia and improved quality of life, although reductions in HbA1c levels have not always been shown, perhaps due to differing participant populations.
Despite the positive outcomes of DAFNE in Australia, funding for this type of intensive, structured education program remains difficult to secure. Limited support for group education is available to people with type 2 diabetes.16 However, the current Medicare Benefits Schedule rebate of $16.00 per group service (Item 81105) is clearly inadequate to fund an intensive education program, and T1DM is excluded. Many OzDAFNE centres have been charging no or minimal fees for the DAFNE course. This allows some people with T1DM to access the program, but limits the number of courses that centres are able to provide.
1 Comparison of baseline characteristics of participants in the DAFNE program who were included and excluded from this study
4 Major clinical outcomes for participants 1 year after DAFNE training (n = 145)
Received 28 May 2009, accepted 3 February 2010
- H David McIntyre1
- Brigid A Knight2
- Dianne M Harvey3
- Marina N Noud2
- Virginia L Hagger3
- Kristen S Gilshenan2
- 1 Endocrinology and Obstetric Medicine, University of Queensland and Mater Health Services, Brisbane, QLD.
- 2 Mater Health Services, Brisbane, QLD.
- 3 Diabetes Australia, Victoria, Melbourne, VIC.
David McIntyre has received speaker fees and travel assistance from companies involved in provision of insulin/delivery systems for type 1 diabetes care, including Novo Nordisk, Eli Lilly, sanofi-aventis and Medtronic. He is a previous President of the DAFNE Association of Australia Inc (now delisted) and Director of a diabetes centre involved in the provision of DAFNE courses, but has derived no personal profit from this.
- 1. DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002; 325: 746.
- 2. Mühlhauser I, Bruckner I, Berger M, et al. Evaluation of an intensified insulin treatment and teaching programme as routine management of type 1 (insulin-dependent) diabetes. The Bucharest-Düsseldorf study. Diabetologia 1987; 30: 681-690.
- 3. National Health and Medical Research Council. When does quality assurance in health care require independent ethical review? Canberra: NHMRC, 2003.
- 4. Shaban MC, Fosbury J, Kerr D, Cavan DA. The prevalence of depression and anxiety in adults with Type 1 diabetes. Diabet Med 2006; 23: 1381-1384.
- 5. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med 2003; 20: 69-72.
- 6. Sämann A, Mühlhauser I, Bender R, et al. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia 2005; 48: 1965-70.
- 7. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977-986.
- 8. Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997; 46: 271-286.
- 9. Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: a meta-analysis. Diabet Med 1997; 14: 919-928.
- 10. Davis RE, Morrissey M, Peters JR, et al. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin 2005; 21: 1477-1483.
- 11. National Association of Diabetes Centres. ANDIAB 2006 — Australian national diabetes information audit and benchmarking. Final report. Canberra: Australian Government Department of Health and Ageing, 2007.
- 12. National Institute for Clinical Excellence. Guidance on the use of patient-education models for diabetes. (Technology appraisal guidance 60.) London: NICE, 2003.
- 13. Shearer A, Bagust A, Sanderson D, et al. Cost-effectiveness of flexible intensive insulin management to enable dietary freedom in people with Type 1 diabetes in the UK. Diabet Med 2004; 21: 460-467.
- 14. Lowe J, Linjawi S, Mensch M, et al. Flexible eating and flexible insulin dosing in patients with diabetes: results of an intensive self-management course. Diabetes Res Clin Pract 2008; 80: 439-443.
- 15. Bendik CF, Keller U, Moriconi N, et al. Training in flexible intensive insulin therapy improves quality of life, decreases the risk of hypoglycaemia and ameliorates poor metabolic control in patients with type 1 diabetes. Diabetes Res Clin Pract 2009; 83: 327-333.
- 16. Australian Government Department of Health and Ageing. Medicare Benefits Schedule. Allied Health Services. 1 May 2010. Part 4. Allied health group services for patients with type 2 diabetes. http://www.health.gov.au/internet/mbsonline/publishing.nsf/Content/FD65645DA9682F63CA2576F60002194F/$File/201005-Allied.pdf (accessed 20 Apr 2010).





Abstract
Objective: To audit and describe the effects of participation in the Dose Adjustment for Normal Eating (DAFNE) course on clinical outcomes in people with type 1 diabetes mellitus (T1DM).
Design, setting and participants: Audit of clinical outcomes before and 1 year after DAFNE training for 145 people with T1DM who participated in courses at seven Australian diabetes centres between February 2005 and March 2007. Participants had been diagnosed with T1DM at least 1 year before and were beyond the “honeymoon phase”, with glycated haemoglobin (HbA1c) < 12% and no severe diabetes complications. They were aged over 17 years and able to understand written and spoken English.
Intervention: A 5-day structured education program covering T1DM management with an emphasis on unrestricted diet, precise carbohydrate estimation and prandial insulin dosing using insulin-to-carbohydrate ratios.
Main outcome measures: Glycaemic control (HbA1c levels), weight, severe hypoglycaemia, and quality of life scores on general (Hospital Anxiety and Depression) and diabetes-specific (Problem Areas in Diabetes) scales.
Results: Mean HbA1c fell from 8.2% to 7.8% (95% CI for change, − 0.5% to − 0.2%; P < 0.0001) and weight from 75.1 to 74.2 kg (95% CI for change, − 1.6 to − 0.2 kg; P = 0.012). Severe hypoglycaemia was less frequent after DAFNE training (P = 0.0001). Quality of life improved (P < 0.0001 for both scales).
Conclusions: One year after participation in the DAFNE program of structured education, people with T1DM showed improved glycaemic control, reduced incidence of severe hypoglycaemia, slightly reduced weight and improved quality of life. The DAFNE course offers one means of improving clinical outcomes in T1DM.