The difficulty in recognising PCG lies partly in its rarity; PCG has a reported incidence of 1 in 30 000 live births in Australia.2 Congenital glaucoma can also be mistaken for a number of common, benign conditions: conjunctivitis, corneal injury, in-turning eyelashes from associated conditions such as epiblepharon (a congenital anomaly in which a fold of skin lies across the lower lid margin), or, as in this case, congenital nasolacrimal duct obstruction. The significance of our patient’s squint is uncertain, although most likely it was a result of her deteriorating visual function.
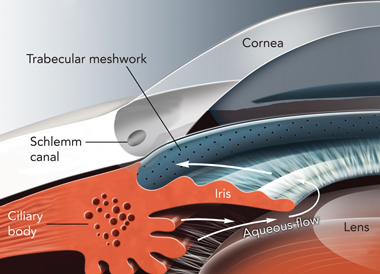
The pathophysiology of PCG appears to be dysgenesis of the trabeculum or its surrounding structures, which results in impaired aqueous outflow and increased intraocular pressure (IOP).3 The conventional outflow path for aqueous humour is shown in the Box. Elevated IOP results in breakdown of corneal endothelial function and an influx of aqueous humour into the normally anhydrous corneal stroma. Prolonged elevation of IOP results in excavation and undermining of the neural and connective tissue of the optic disc, and the development of optic neuropathy.
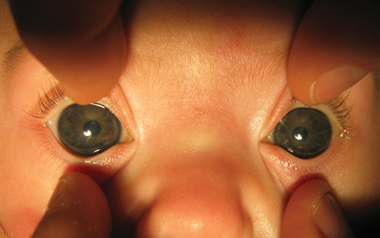
The first clinical signs of PCG include the triad of blepharospasm, photophobia and excessive tearing. Progressively, the corneas become oedematous, lose clarity, and, if untreated, may become opaque. Elevated IOP in an immature eye also results in progressive enlargement of the cornea and sclera, known as buphthalmos (“ox eye”). Potential for corneal enlargement generally ceases when the child reaches 3 years of age, although scleral enlargement can increase beyond this age.3,4 The horizontal corneal diameter in full-term newborn infants averages 9.8 mm; a corneal diameter greater than 11 mm is enlarged.5 A horizontal corneal diameter greater than 12 mm in a 12-month-old child suggests abnormality.6 Progressive enlargement of the cornea may also result in horizontal breaks in the basement membrane of the corneal endothelium (Descemet membrane), known as Haab striae.3
Lessons from practice
Congenital glaucoma is a sight-threatening differential diagnosis in an infant who presents with watering eyes.
Key clinical features of congenital glaucoma are a cloudy cornea, excessive tearing, blepharospasm, photophobia and an enlarged globe.
In infants, corneal size is a surrogate marker of intraocular pressure. Enlargement and asymmetry in corneal diameter requires further investigation.
Patients with suspected congenital glaucoma require urgent referral to an ophthalmologist.
The mean age of diagnosis is 4.4 months.2 Severe disease is occasionally observed in neonates, although in its early stages PCG tends to have few signs. An urgent referral to an ophthalmologist is required if tearing is associated with photophobia or blepharospasm; the cornea is hazy; or the eye or cornea is enlarged or asymmetrical. It is important to distinguish PCG from congenital nasolacrimal duct obstruction: infants with the latter also have excessive tearing, but, in contrast to PCG, the cornea remains clear, there is no enlargement of the globe and no photophobia or blepharospasm. Nasolacrimal duct obstruction is common, typically innocuous, and frequently resolves spontaneously within the first 12 months of life. It can be safely assessed and managed by the primary-care physician.
Surgery is the first-line treatment for PCG. The two most common procedures are goniotomy and trabeculotomy, both of which involve microsurgical dissection of the trabecular meshwork, with the aim of increasing aqueous outflow. Ocular antihypertensive agents are an important adjunct to surgery, and are often used as a temporising measure. Screening for amblyopia and correction of asymmetrical refractive errors are undertaken until the child’s vision is developmentally mature to ensure the best visual outcome achievable for the child. Lifelong follow-up is often required. With modern treatment, a visual outcome of better than 6/15 is achieved in up to 79% of cases of PCG; whereas, if untreated, this condition carries an exceedingly poor visual prognosis.3,7
- 1. Dominguez A, Banos MS, Alvarez MG, et al. Intraocular pressure measurement in infants under general anaesthesia. Am J Ophthalmol 1974; 78: 110-116.
- 2. MacKinnon JR, Giubilato A, Elder JE, et al. Primary infantile glaucoma in an Australian population. Clin Experiment Ophthalmol 2004; 32: 14-18.
- 3. Beck AD. Diagnosis and management of pediatric glaucoma. Ophthalmol Clin North Am 2001; 14: 501-512.
- 4. Waring GO, Laibson PR, Rodrigues M. Clinical and pathological alterations of Descemet’s membrane: with emphasis on endothelial metaplasia. Surv Ophthalmol 1974; 18: 325-368.
- 5. Goes F. Ocular biometry in childhood. Bull Soc Belge Ophtalmol 1982; 202: 159-193.
- 6. Childhood glaucoma. In: American Academy of Ophthalmology. Basic and clinical science course: glaucoma. San Francisco: AAO, 2005: 147-154.
- 7. Richardson KT Jr, Ferguson WJ Jr, Shaffer RN. Long-term functional results in infantile glaucoma. Trans Am Acad Ophthalmol Otolaryngol 1967; 71: 833-837.





None identified.