Opioid substitution treatment — administering a substitute opioid to help manage withdrawal or maintain a patient on a controlled dose — aims to reduce illicit opioid use and its attendant harms.1-4 Non-adherence with directions for taking opioid substitution treatment medication includes injection when it is not prescribed as injectable, and diversion for injection by a person for whom it is not prescribed.5,6 Both injection and diversion of pharmaceutical opioids (including those prescribed for pain management) cause significant public health problems in many countries.7-10 However, few strategies to deter injection or reduce diversion have been empirically evaluated.
In Australia, methadone is the most widely used opioid substitution treatment, but since November 2000, higher-dose formulations of buprenorphine (BPN; Subutex, Reckitt Benckiser) have been registered for this purpose. Implementation of BPN is largely based on that used for methadone (Box 1), but with very restricted availability of takeaway (unsupervised) doses, because of the problems of injection and diversion. Injection of BPN sublingual tablets has been associated with limb ischaemia and tissue necrosis, abscesses, cellulitis, endocarditis, nerve damage, thrombosis, pulmonary granuloma, and candida endophthalmitis.9,12-14
Diversion and injection of opioid substitution treatment medications vary according to prescribing, client supervision and dispensing practices; cost of treatment; the availability of other drugs; and cultural factors.10,15 There is suggestive evidence that supervised dosing, and both dilution of methadone takeaways and restricted access to syringes facilitating injection of large volumes, may reduce diversion and injection, respectively.16 To deter injection, a new formulation of opioid substitution treatment has been developed — buprenorphine (a partial opioid agonist) combined with naloxone (an opioid antagonist) in a 4 : 1 ratio (BNX; Suboxone, Reckitt Benckiser).17,18 When taken sublingually, BNX’s actions are indistinguishable from BPN alone. However, when injected by a person dependent on a full agonist, such as heroin or methadone (but who is not in withdrawal), BNX can precipitate a more aversive withdrawal syndrome than injecting BPN alone.17,19 There is also limited laboratory evidence that it can be injected by those already taking BPN without causing withdrawal features.20
The Illicit Drug Reporting System (IDRS) is an established program of research that monitors trends in Australian illicit drug use and markets.11,21 It includes interviews with regular IDUs (about 900 each year) who are selected because of their involvement in central inner-city drug markets in Australian capital cities. We included data from the 2003–2007 IDRS interviews with IDUs.
National monthly sales data for BPN and BNX were provided by Reckitt Benckiser, who also provided commercially available data on sales of methadone liquid formulations (Methadone syrup [GlaxoSmithKline] and Biodone [National Sales Solutions]). Sales data were expressed in “factored units” of average doses of methadone in Australia, assumed to be 70 mg, and average doses of buprenorphine (BPN or BNX), assumed to be 12 mg; these levels are derived from previous research on client doses in Australia.22,23
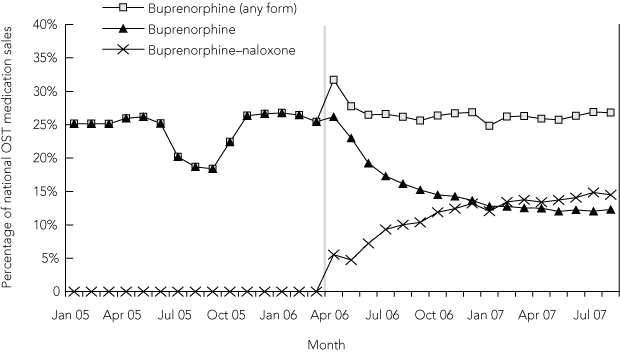
The proportion of opioid substitution treatment medication sales in Australia accounted for by BPN and BNX is shown in Box 2. There was a steady increase in sales of BNX from April 2006; by August 2007, it accounted for more of the total Australian opioid substitution treatment medication sales than BPN. The total buprenorphine (BPN plus BNX) market share did not change: it consistently accounted for just over 25% of all doses (with methadone accounting for the remainder).
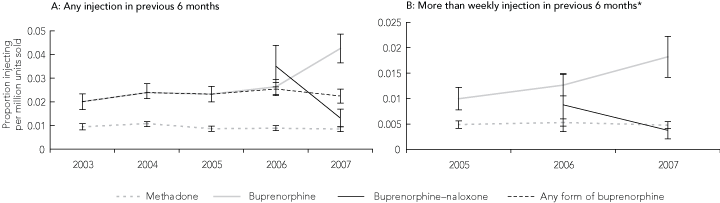
Box 3 presents data on injection of the three opioid substitution treatment medications by regular IDUs in Australian capital cities, adjusting for the volume of sales of each medication type (expressed as the ratio of the proportions injecting in the previous 6 months per 100 million units sold in the same period). Injection of methadone includes Methadone syrup, Biodone and Physeptone (GlaxoSmithKline). The data were derived from the total samples of IDUs in each year reporting injection of each type of opioid substitution treatment in the previous 6 months.
Box 4 shows the proportions of (i) regular IDUs not in any form of drug treatment, and (ii) clients currently in each form of opioid substitution treatment, who reported any and more than weekly injection of each opioid substitution treatment medication during the 6 months before interview. The former group represent a group injecting someone else’s medication (ie, diversion); the latter represent a group presumed to be injecting their own medication (ie, non-adherence).
The results of multiple regression analysis of predictors of recent injection of opioid substitution treatment medications are shown in Box 5. In both clients and IDUs, the strongest predictors were the recent injection of other pharmaceutical opioids. Clients and IDUs who injected one type of medication were likely to inject a range of medications. Days of heroin use was not related to injection of opioid substitution treatment, and, for clients, nor was length of time in treatment. However, there were jurisdictional differences in the levels of injection of opioid substitution treatment medications. For example, IDUs from Western Australia and Queensland were 27.5 and 20.3 times, respectively, more likely to inject BNX than IDUs from NSW, reflecting lower levels of BNX prescribing in NSW.
We were able to assess changes in the levels of injection of opioid substitution treatment medications among at-risk IDUs in all Australian capital cities, adjusting for availability as indicated by sales data, and allowing for direct comparisons of three opioid substitution treatment medications. These data suggest that BNX is less likely to be regularly injected than either methadone or BPN, consistent with laboratory studies.24
Given that BNX has not only overtaken market sales of BPN, but is also generally available as takeaway medication (unlike BPN), the deterrent effect of the combination product may be even greater than the comparisons in our study suggest. This finding has important implications for public health, given the potential for severe consequences of BPN injection. We acknowledge that particular local factors (eg, treatment access and the illicit drug market) will also exert a strong influence on non-adherence and injection. Nevertheless, our findings, together with those showing that weekly dispensed BNX treatment has comparable efficacy to and greater cost-effectiveness than daily supervised BPN,25 suggest that BNX may be expanded as a safe and effective treatment, with perhaps less requirement for supervised dispensing.
Despite the lower levels of injection, we documented that BNX was injected by IDUs and clients. Thus, even with a formulation designed to deter injection, there continues to be some level of non-adherence and injection. Many factors independent of medication formulation are likely to influence the extent of diversion and injecting, including availability of heroin and policies allowing multiple takeaway doses, as well as client characteristics. Client selection to ensure takeaway doses are restricted to those who have largely ceased injecting and have social stability is the basis of the current Australasian Chapter of Addiction Medicine’s Clinical Guidelines.26 This recommendation is consistent with studies suggesting that the people most likely to inject BPN are more chaotic and socially marginalised.27,28
There was clear evidence in our study that IDUs and clients who were injecting other pharmaceutical opioids were also more likely to inject opioid substitution treatment medication, suggesting a preference for injection, even if the medications are not designed to be injected. The role of injectable opioids as a form of opioid substitution treatment has been hotly debated. There is now evidence showing the feasibility and effectiveness of injectable opioid treatment in reducing unsanctioned drug use and related crime, and in enhancing general health and psychosocial functioning.29 The propensity for this group to inject existing pharmaceutical opioids (eg, methadone, BPN, morphine) suggests that these medications may be more feasible alternatives to establishing a heroin treatment program, with all its complex medicolegal hurdles. A randomised controlled trial comparing injectable methadone, injectable heroin and oral methadone is currently underway in the United Kingdom.30
1 Opioid substitution treatment in Australia
Opioid substitution treatment has been available in Australia for 15 years; treatment coverage is relatively good, with over 30 000 people receiving treatment.11 Methadone, buprenorphine, and buprenorphine–naloxone are the medications used.
2 Estimated proportion of opioid substitution treatment (OST) medication sales accounted for by buprenorphine and buprenorphine–naloxone, January 2005 – August 2007
3 Ratio of injection of opioid substitution treatment (OST) medications by regular injecting drug users to volume of sales of OST medications, 2003–2007
 | |||||||||||||||
4 Methadone, buprenorphine and buprenorphine–naloxone injection among regular injecting drug users (IDUs) currently not being treated, and clients enrolled in opioid substitution treatment (OST) in 2007
5 Predictors of injection in the previous 6 months of opioid substitution treatment (OST) medication (odds ratios [95% CIs]) among regular injecting drug users (IDUs) currently not being treated, and clients enrolled in OST
Received 11 August 2008, accepted 15 January 2009
- Louisa Degenhardt1
- Briony K Larance1
- James R Bell2
- Adam R Winstock3
- Nicholas Lintzeris3
- Robert L Ali4
- Nicolas Scheuer1
- Richard P Mattick1
- 1 National Drug and Alcohol Research Centre, University of New South Wales, Sydney, NSW.
- 2 The Langton Centre, South Eastern Sydney and Illawarra Area Health Service, Sydney, NSW.
- 3 Drug Health Services, Sydney South West Area Health Service, Sydney, NSW.
- 4 WHO Collaborating Centre for the Treatment of Drug and Alcohol Problems, University of Adelaide, Adelaide, SA.
We thank the many people receiving opioid substitution treatment, or who regularly inject drugs, who agreed to be interviewed; and Susannah O’Brien for her assistance with coordination of this project.
Louisa Degenhardt, Briony Larance and Richard Mattick were provided with an untied educational grant by Reckitt Benckiser to monitor the extent of injection of BNX, and to compare it with injection of established opioid substitution treatment medications; they and Adam Winstock have received support for speaking at workshops held by Reckitt Benckiser. Reckitt Benckiser had no role in the design, conduct, reporting, or analysis of the study, or in interpretation of the study results or preparation of the manuscript. James Bell and Robert Ali have previously received support for speaking at workshops held by Reckitt Benckiser and Schering Plough.
- 1. World Health Organization/United Nations Office of Drugs and Crime/Joint United Nations Programme on HIV/AIDS. WHO/UNODC/UNAIDS position paper: substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention. Geneva: WHO, 2004.
- 2. Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2008; (2): CD002207.
- 3. Mattick RP, Kimber J, Breen C, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2003; (2): CD002209.
- 4. NHS National Institute for Health and Clinical Excellence. Methadone and buprenorphine for managing opioid dependence. NICE Technology Appraisal Guidance 114. London: NICE, 2007.
- 5. Inciardi JA, Surratt H, Kurtz SP, Burke JJ. The diversion of prescription drugs by health care workers in Cincinnati, Ohio. Subst Use Misuse 2006; 41: 255-264.
- 6. Inciardi JA, Surratt HL, Kurtz SP, Cicero TJ. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med 2007; 8: 171-183.
- 7. Rajagopal MR, Joranson DE, Gilson AM. Medical use, misuse, and diversion of opioids in India. Lancet 2001; 358: 139-143.
- 8. Parfitt T. Designer drug Subutex takes its toll in Tbilisi. Lancet 2006; 368: 273-274.
- 9. Jenkinson R, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction 2005; 100: 197-205.
- 10. Lintzeris N, Lenne M, Ritter A. Methadone injecting in Australia: a tale of two cities. Addiction 1999; 94: 1175-1178.
- 11. O’Brien S, Black E, Roxburgh A, et al. Australian drug trends 2006: findings from the Illicit Drug Reporting System (IDRS). NDARC Monograph 60. Sydney: National Drug and Alcohol Research Centre, University of New South Wales, 2007.
- 12. Degenhardt L, Larance B, Mathers B, et al. Benefits and risks of pharmaceutical opioids: essential treatment and diverted medication. A global review of availability, extra-medical use, injection and the association with HIV. Sydney: National Drug and Alcohol Research Centre, University of New South Wales, 2008.
- 13. Aboltins CA, Daffy JR, Allen P. Fungal endophthalmitis in intravenous drug users injecting buprenorphine contaminated with oral Candida species [letter]. Med J Aust 2005; 182: 427. <MJA full text>
- 14. Winstock AR, Lea T, Sheridan J. Prevalence of diversion and injection of methadone and buprenorphine among clients receiving opioid treatment at community pharmacies in New South Wales, Australia. Int J Drug Policy 2008; 19: 450-458.
- 15. Ritter A, Di Natale R. The relationship between take-away methadone policies and methadone diversion. Drug Alcohol Rev 2005; 24: 347-352.
- 16. Humeniuk R, Ali R, McGregor C, Darke S. Prevalence and correlates of intravenous methadone syrup administration in Adelaide, Australia. Addiction 2003; 98: 413-418.
- 17. Bell J, Byron G, Gibson A, Morris A. A pilot study of buprenorphine-naloxone combination tablet (SuboxoneTM) in the treatment of opioid dependence. Drug Alcohol Rev 2004; 23: 311-317.
- 18. McAleer SD, Mills RJ, Polack T, et al. Pharmacokinetics of high-dose buprenorphine following single administration of sublingual tablet formulations in opioid naive healthy male volunteers under a naltrexone block. Drug Alcohol Depend 2003; 72: 75-83.
- 19. Ling W, Smith D. Buprenorphine: blending practice with research. J Subst Abuse Treat 2002; 23: 87-92.
- 20. Harris D, Jones RT, Welm S, et al. Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend 2000; 61: 85-94.
- 21. Topp L, McKetin R. Supporting evidence-based policy-making: a case study of the IDRS. Bulletin on Narcotics 2003; LV, Nos 1 and 2: 23-30.
- 22. Winstock AR, Lea T, Madden A, Bath N. Know-+ledge about buprenorphine and methadone among those receiving treatment for opioid dependence. Drugs Educ Prev Policy 2008; 15: 395-409.
- 23. Lintzeris N, Pritchard E, Sciacchitano L. Investigation of methadone dosing in Victoria: factors influencing dosing levels. Melbourne: Turning Point Alcohol and Drug Centre, 2007.
- 24. Stoller KB, Bigelow GE, Walsh S, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology 2001; 154: 230-242.
- 25. Bell J, Shanahan M, Mutch C, et al. A randomized trial of effectiveness and cost-effectiveness of observed versus unobserved administration of buprenorphine-naloxone for heroin dependence. Addiction 2007; 102: 1899-1907.
- 26. Royal Australasian College of Physicians Australasian Chapter of Addiction Medicine. Clinical guidelines: assessing suitability for unsupervised medication doses in the treatment of opioid dependency. http://www.racp.edu.au/page/about-the-racp/structure/australasian-chapter-of-addiction-medicine (accessed Apr 2009).
- 27. Vidal-Trecan G, Varescon I, Nabet N, Boissonnas A. Intravenous use of prescribed sublingual buprenorphine tablets by drug users receiving maintenance therapy in France. Drug Alcohol Depend 2003; 69: 175-181.
- 28. Guichard A, Lert F, Calderon C, et al. Illicit drug use and injection practices among drug users on methadone and buprenorphine maintenance treatment in France. Addiction 2003; 98: 1585-1597.
- 29. Haasen C, Verthein U, Degkwitz P, et al. Heroin-assisted treatment for opioid dependence: randomised controlled trial. Br J Psychiatry 2007; 191: 55-62.
- 30. Lintzeris N, Strang J, Metrebian N, et al; RIOTT Group. Methodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable methadone and injectable heroin treatment versus optimised oral methadone treatment in the UK. Harm Reduct J 2006; 3: 28.





Abstract
Objectives: To examine the levels and predictors of injection of buprenorphine–naloxone (BNX) — a combination of a partial opioid agonist and an opioid antagonist for treating opioid dependence — which was specifically developed to limit injecting. Comparison was made with injecting of two other opioid substitution treatment medications, methadone and buprenorphine (BPN); severe harms have been documented after injection of the latter.
Design and participants: Injecting was studied in regular injecting drug users (“IDUs”) and current opioid substitution treatment clients (“clients”). Regular IDUs are interviewed annually in each Australian capital city (about 900 per year) and data for 2003–2007 were used; 399 clients were interviewed in 2007. Data on injection of opioid substitution treatment medications between 2003 and 2007 were adjusted for availability of medications (from national sales data for methadone, BPN and BNX). Predictors of injecting were analysed by multiple regression analyses.
Setting: Capital cities of all Australian states and territories.
Main outcome measure: Injection of opioid substitution treatment medications among individuals both in and out of treatment.
Results: In the year after its introduction in Australia, BNX was injected less frequently and by fewer regular IDUs and clients compared with BPN, particularly when differences in the availability of medications were taken into account. Some individuals did nonetheless regularly inject BNX. Injection of methadone, BPN and BNX was more likely to occur among those injecting other pharmaceutical opioids.
Conclusions: A partial opioid agonist–antagonist combination appears to be less commonly and less frequently injected by clients in treatment and IDUs who are not. Further studies are needed to evaluate longer-term trends in use and harms.