Rotavirus is the most common global cause of severe early childhood gastroenteritis and has a significant clinical impact.1 In the pre-vaccine era, it was responsible for about 10 000 hospitalisations, 22 000 emergency department presentations and 115 000 general practice consultations annually in Australian children less than 5 years of age.2
To reduce this substantial disease burden, two rotavirus vaccines have been licensed for use in Australian infants: RotaTeq (Merck, Whitehouse Station, NJ, USA), a live multivalent bovine–human reassortant vaccine;3 and Rotarix (GlaxoSmithKline, Rixensart, Belgium), a single-strain live-attenuated human vaccine.4 Australian infants have been eligible for rotavirus vaccination via the National Immunisation Program since July 2007.5 Queensland children born on or after 1 May 2007 are eligible for three publicly funded doses of RotaTeq. Both vaccines were available on the private market from 2006, but, as with other recommended but non-funded vaccines,6 uptake is likely to have been modest.
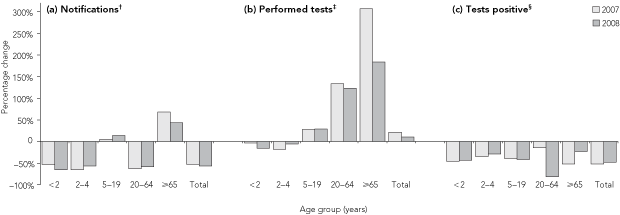
Annual rotavirus notifications have fallen since the introduction of public funding for universal infant vaccination: compared with 2510 notifications in 2006, there were 1189 notifications in 2007 (a 53% reduction) and 1087 notifications in 2008 (a 57% reduction) (Box 1, a). In children less than 2 years of age, the reduction was 53% in 2007 and 65% in 2008, with reductions of similar magnitude in children aged 2–4 years (65% and 56%, respectively).
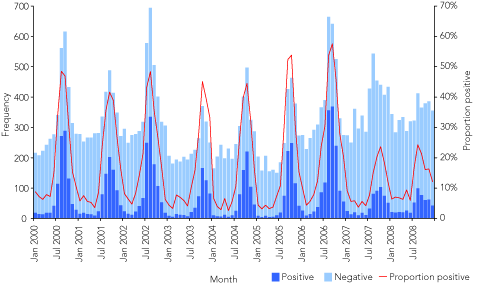
The number of tests performed and the proportion of all tests positive for rotavirus in Queensland had an annual winter–spring peak (Box 2). Comparing the mean annual number of rotavirus tests performed in the years 2000–2006 with values for 2007 and 2008 revealed variation between age groups: there was a fall in the number of tests performed for children aged less than 2 years (–3% [2007]; –15% [2008]) and aged 2–4 years (–18% [2007]; –5% [2008]), but an increase in tests performed for all older age groups (Box 1, b). The total number of tests increased from a mean annual figure of 3720 (over the period 2000–2006) to 4511 in 2007 and 4113 in 2008.
Before public funding of the universal infant vaccination program, the annual peak monthly value of the proportion of tests positive ranged from a low of 42% (September 2001) to a high of 58% (September 2006). After introduction of public funding, peak monthly values dropped to 24% (in both October 2007 and August 2008) (Box 2). In every age group (ie, including non-vaccinated age groups) there was a reduction in the proportion of tests that were positive in 2007 and 2008 compared with the proportion over the period 2000–2006 (Box 1, c). In children less than 2 years of age, reductions in the proportion of tests positive (relative to the proportion in 2000–2006) were 45% in 2007 and 43% in 2008.
Concordant findings have been seen elsewhere: rotavirus activity was delayed by up to 4 months and diminished in size by more than 50% in the United States after the introduction of the RotaTeq vaccine,8 and there was evidence of vaccine effectiveness during a recent outbreak in the Northern Territory, where the Rotarix vaccine is used.9
Our Queensland data are an important addition to current findings, as they are among the first to show changes in rotavirus epidemiology in non-vaccinated age groups,10 providing good evidence for an equivalent indirect vaccine effect in older age groups. These local outcomes were achieved within 18 months of the program’s commencement. Of the first two eligible birth cohorts in which coverage was measured, 75% of infants born in May–July 2007 and 80% of those born in August–September 2007 had received a complete three-dose vaccine course on assessment at 12 months of age (Mr Brynley Hull, Epidemiologist, National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases, personal communication). Improved benefits are anticipated as more birth cohorts are vaccinated and more courses completed.
Our only divergent finding was the increase in the number of tests performed for rotavirus in older age groups. The request slip audit showed that in the oldest age group there was less likely to be a specific request for rotavirus or adenovirus testing, and that the proportion of rotavirus tests done in response to a request for non-specific viral testing was higher. There has recently been an increased awareness of viral gastroenteritis outbreaks in hospitals, nursing homes and residential aged-care facilities.11,12 It may be that, in some circumstances, rotavirus testing is being done routinely on gastroenteritis specimens from older people, even though the overall number and proportion of specimens found positive is likely to be low.
Our data come from two passive, routinely collected sources. Both are likely to be reasonably representative of all regions in Queensland, and the data from 33 Queensland Health laboratories are likely to include nearly all testing done in rural and remote locations, which are not as well served by private pathology companies. It is worth keeping in mind that our results are produced from observational studies relating trends in rotavirus outcome data before and after vaccine introduction: this is a less robust study design than other observational studies for assessing a causal relationship. The documented changes may represent secular trends — however, the results would represent unusual, persisting and extreme temporal variations. Further, the reductions seen coincide closely with vaccine introduction, and are in keeping with both US post-licensure data8 and vaccine efficacy studies showing a 98%–100% reduction in severe rotavirus gastroenteritis and a 73%–74% reduction in any rotavirus gastroenteritis.3,13 To support the role of vaccination in these locally improving trends, better quality observational studies are required, including efforts to estimate vaccine effectiveness using routinely collected data14 and during identified outbreaks.9 Australia, being in the unique position of having widespread and region-specific use of both currently licensed rotavirus vaccines, could further add to global research by routinely monitoring the field effectiveness of each vaccine, as well as regional differences in circulating genotypes, through the National Rotavirus Reference Centre.15
Received 28 January 2009, accepted 30 April 2009
- Stephen B Lambert1
- Cassandra E Faux1
- Lisa Hall2
- Frances A Birrell2
- Karen V Peterson2
- Christine E Selvey2
- Theo P Sloots1,3,4
- Michael D Nissen5
- Keith Grimwood1,4,5
- 1 Queensland Paediatric Infectious Diseases Laboratory, Royal Children’s Hospital, Brisbane, QLD.
- 2 Communicable Diseases Branch, Queensland Health, Brisbane, QLD.
- 3 Clinical and Statewide Services, Queensland Health, Brisbane, QLD.
- 4 Discipline of Paediatrics and Child Health, University of Queensland, Brisbane, QLD.
- 5 Royal Children’s Hospital, Brisbane, QLD.
We would like to thank Brynley Hull and Peter McIntyre from the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases for providing Queensland rotavirus vaccine coverage figures from the Australian Childhood Immunisation Register.
Stephen Lambert has been an investigator on industry-sponsored vaccine studies, has received support for conference attendance from GlaxoSmithKline and CSL, and been a member of vaccine advisory boards for GlaxoSmithKline and Novartis. Christine Selvey has previously received support from CSL to attend a local meeting. Michael Nissen has been an investigator on industry-sponsored studies and has received support from Wyeth and GlaxoSmithKline for conference attendance. Keith Grimwood was a member of a rotavirus vaccine advisory board and received support for conference attendance, lecture fees, and a research grant from GlaxoSmithKline, as well as a research grant from Merck. The research for this article was done entirely within the authors’ roles in Queensland Health, and was not funded by any external grant or agency.
- 1. Grimwood K, Lambert SB. Rotavirus vaccines: opportunities and challenges. Hum Vaccin 2009; 5: 4-16.
- 2. Galati JC, Harsley S, Richmond P, Carlin JB. The burden of rotavirus-related illness among young children on the Australian health care system. Aust N Z J Public Health 2006; 30: 416-421.
- 3. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354: 23-33.
- 4. Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354: 11-22.
- 5. Australian Government Department of Health and Ageing. Fact sheet: Australian Government funding of rotavirus vaccine. http://www.health.gov.au/internet/main/publishing.nsf/Content/rotavirus_vaccine.htm (accessed Apr 2009).
- 6. Gilbert GL, Gidding HF, Backhouse J, et al. Varicella seroprevalence and vaccine uptake in preschool children [letter]. Med J Aust 2005; 182: 42. <MJA full text>
- 7. National Health and Medical Research Council, Australian Research Council and Australian Vice-Chancellors’ Committee. National statement on ethical conduct in human research. Canberra: Australian Government, 2007. http://www.nhmrc.gov.au/PUBLICATIONS/synopses/_files/e72.pdf (accessed Apr 2009).
- 8. Centers for Disease Control and Prevention. Delayed onset and diminished magnitude of rotavirus activity — United States, November 2007–May 2008. MMWR Morb Mortal Wkly Rep 2008; 57: 697-700.
- 9. Graham J, Cook H, Roberts C, et al. An estimate of rotavirus vaccine efficacy following an outbreak of rotavirus gastroenteritis in Central Australia. Northern Territory Dis Control Bull 2008; 15: 8-13.
- 10. Lieberman JM, Huang X, Koski E, et al. Decline in rotavirus cases in the US after licensure of a live, oral rotavirus vaccine. 48th Annual Meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of America; 2008 Oct 25–28; Washington, DC, USA: G1-G437.
- 11. Lyon MJ, Wei G, Smith GA. Epidemic viral gastroenteritis in Queensland coincides with the emergence of a new norovirus variant. Commun Dis Intell 2005; 29: 370-373.
- 12. Kirk MD, Roberts L, Horvath J. Understanding gastroenteritis in elderly residents of aged-care facilities [editorial]. Med J Aust 2008; 189: 476-477. <MJA full text>
- 13. Block SL, Vesikari T, Goveia MG, et al. Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics 2007; 119: 11-18.
- 14. Torvaldsen S, McIntyre PB. Observational methods in epidemiologic assessment of vaccine effectiveness. Commun Dis Intell 2002; 26: 451-457.
- 15. Kirkwood CD, Cannan D, Bogdanovic-Sakran N, et al. Australian Rotavirus Surveillance Program: annual report, 2006–07. Commun Dis Intell 2007; 31: 375-379.





Abstract
Objective: To assess the impact of introducing a publicly funded infant rotavirus vaccination program on disease notifications and on laboratory testing and results.
Design and setting: Retrospective analysis of routinely collected data (rotavirus notifications [2006–2008] and laboratory rotavirus testing data from Queensland Health laboratories [2000–2008]) to monitor rotavirus trends before and after the introduction of a publicly funded infant rotavirus vaccination program in Queensland in July 2007.
Main outcome measures: Age group-specific rotavirus notification trends; number of rotavirus tests performed and the proportion positive.
Results: In the less than 2 years age group, rotavirus notifications declined by 53% (2007) and 65% (2008); the number of laboratory tests performed declined by 3% (2007) and 15% (2008); and the proportion of tests positive declined by 45% (2007) and 43% (2008) compared with data collected before introduction of the vaccination program. An indirect effect of infant vaccination was seen: notifications and the proportion of tests positive for rotavirus declined in older age groups as well.
Conclusions: The publicly funded rotavirus vaccination program in Queensland is having an early impact, direct and indirect, on rotavirus disease as assessed using routinely collected data. Further observational studies are required to assess vaccine effectiveness. Parents and immunisation providers should ensure that all Australian children receive the recommended rotavirus vaccine doses in the required timeframe.