Hepatitis C virus (HCV) infection is a major cause of chronic liver disease, including cirrhosis and liver cancer. It is estimated that about 3% of the world’s population is infected with HCV.1 In Australia, it is estimated that 264 000 people were seropositive for HCV in 2005.2 Transmission of HCV in Australia occurs mainly through intravenous drug use. Depending on factors such as duration of injecting, age and sex, the proportion of intravenous drug users who are seropositive for HCV has been estimated to be 50%–75%.3-5
Mother-to-infant transmission, or vertical transmission, of HCV occurs infrequently,6 but is a cause for concern. This is because of possible chronic HCV infection and progression to cirrhosis and hepatocellular carcinoma later in life, as well as occasional development of end-stage liver disease in childhood.7
Vertical transmission of HCV occurs only when the mother is HCV RNA-positive (ie, viraemic) at the time of birth, with the risk of transmission being 5%–10%.8-10 After transmission, persistent infection develops in at least 85% of infected newborns, even in the absence of biochemical evidence of liver disease.11,12
Screening for HCV infection in the antenatal care setting has been deemed to be important for detecting infection in mothers and identifying infants who are most at risk of vertical transmission. The current NSW Department of Health guidelines recommend that HCV screening be performed for women with risk factors such as a history of intravenous drug use,13 while the current Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) guidelines recommend universal screening of pregnant women regardless of the presence of risk factors.14
The NSW Department of Health and RANZCOG guidelines both recommend that women who are seropositive for HCV be tested by HCV RNA polymerase chain reaction (PCR) to detect viraemia, and that infants of women who test positive for HCV RNA be screened for HCV by serological testing at 18 months of age.13,14 Whether these recommendations are being followed in practice is unknown. The aim of this study was to describe the patterns of HCV screening in pregnant women on methadone maintenance treatment and their infants.
The two metropolitan hospitals have strong clinical links, which facilitated the ethics approval process and data collection. The proportion of pregnant woman who use illicit drugs in pregnancy is very high in the area serviced by these hospitals (approximately 80 pregnant mothers per year, accounting for about 1% of births, according to personal experience of local clinicians); this enabled us to acquire data for a reasonable sample size. According to Australian Bureau of Statistics data, Socio-Economic Indexes for Areas scores for Penrith and Blacktown in 2006 were in the lower deciles.15 Aboriginal and Torres Strait Islanders represented 2.5% of the population in the Penrith local government area16 and 2.7% of the population in the Blacktown local government area,17 compared with 2.2% in NSW overall.18
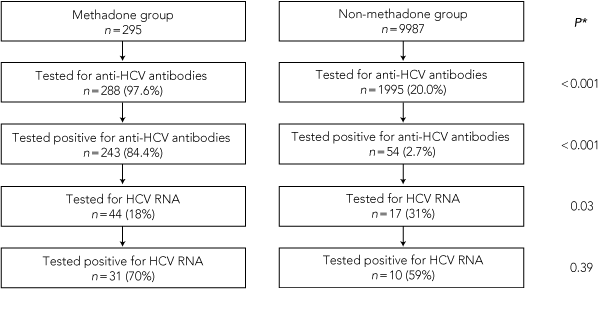
Serological testing for anti-HCV antibodies was performed for 97.6% of women in the methadone group, compared with 20.0% of women in the non-methadone group (P < 0.001). Of women who were seropositive for HCV, those in the methadone group were significantly younger than those in the non-methadone group (27.9 ± 5.4 years v 30.2 ± 6.1 years; P = 0.01). Subsequent HCV RNA testing was performed in 18.1% of HCV-seropositive women in the methadone group, compared with 31.5% of HCV-seropositive women in the non-methadone group (P = 0.03) (Box 1).
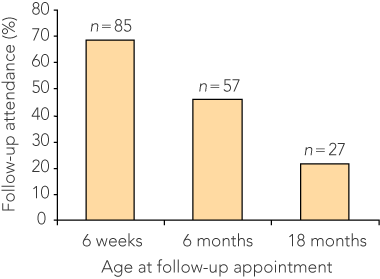
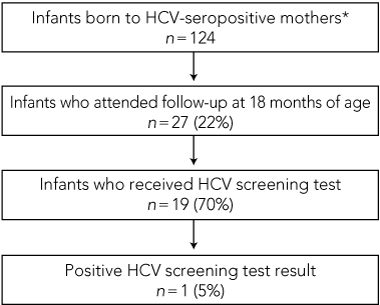
Of 195 infants born to HCV-seropositive mothers at the larger metropolitan hospital, 124 were followed up at various ages (64%) (Box 2). The remainder were lost to follow-up, even though a follow-up appointment was arranged at discharge. Of those 124 infants for whom we had follow-up attendance data, 34 (27%) received some level of screening during the 18 months after birth, and positive results for anti-HCV antibodies and HCV RNA were returned for one infant at 18 months of age.
Twenty-seven of the 124 infants attended the recommended follow-up at 18 months of age (22%), of whom 19 were tested for either anti-HCV antibodies or HCV RNA (70%) (Box 3).
To our knowledge, this is the first study to investigate the seroprevalence of HCV in pregnant women on methadone replacement treatment. Approximately 98% of methadone-maintained pregnant women underwent HCV serological testing, of whom 84% were seropositive. Of those who were seropositive, 18% were subsequently tested for HCV RNA, and 71% of those tested were HCV RNA-positive. This is in keeping with previously published data, which showed that up to 75% of HCV-seropositive individuals with a past history of intravenous drug use are viraemic.19-21
In an article published in 2003, HCV screening practice was shown to be variable — 24 of 62 Australian public hospital-based obstetric units surveyed had a specific antenatal testing policy for HCV (39%), and 14 offered universal antenatal testing for HCV (23%).22 Our data highlight that HCV testing practice during pregnancy is inadequate, as most HCV RNA-positive mothers in our study would have missed testing. This implies that the opportunity for monitoring and future therapy in this high-risk group was lost. Awareness of an infectious maternal HCV status enables counselling and support regarding maternal therapeutic options, as well as caution in terms of preventing transmission to partners.
Inadequate screening was not limited to the methadone-maintained women; screening was also inadequate in the non-methadone group, although the follow-up RNA testing was slightly better. This implies that sociobehavioural factors in the methadone group might affect screening rates. Whatever the reason, it is clear that HCV screening in pregnancy is not adequately followed through. In addition, screening patterns and test results at the rural and metropolitan settings we studied have previously been shown to be similar.23
Our study also showed that about one in five infants born to HCV-seropositive mothers, of those for whom we had follow-up attendance data, attended a follow-up appointment at 18 months of age. Therefore, most infants with the highest known risk for acquiring HCV missed out on testing at the recommended age. In addition, we identified one case of HCV vertical transmission from the 295 methadone-maintained women, of whom 243 were HCV-seropositive. Assuming an approximate prevalence of 75% HCV RNA positivity among HCV-seropositive individuals19-21 and a 5% vertical transmission rate,8-10 we would have missed about 10 cases of vertical HCV transmission. Although the prevalence of HCV infection in children in Australia is unknown, it has been estimated that 75–100 new cases of vertically acquired HCV occur each year. The much lower reported number of cases (27 in 2003, of which 12 were confirmed24) implies that childhood HCV infection is underdiagnosed in Australia.19
There are numerous advantages of HCV screening and identifying patients with HCV infection. HCV is a notifiable disease in Australia, but because most cases of vertically transmitted HCV go undetected, screening would allow the true extent of the problem to become known. It is important to identify HCV-positive children — this enables monitoring of viral activity and disease by clinicians, who might initiate treatment or advise vaccination against other viral hepatitides that may facilitate progression to liver disease.19
Our recommendations regarding screening for HCV infection in pregnant women and their infants, based on our findings, are shown in Box 4.
Current HCV screening practice in the high-risk group of methadone-maintained pregnant women and their infants is inadequate. Failure to identify pregnant women with HCV viraemia leads to a failure to identify infants who are most at risk of vertical transmission. A more consistent approach for maternal RNA testing is required, which may be best achieved by educating health care providers who provide antenatal care to pregnant women who are at high risk of HCV viraemia. In addition, a greater proportion of affected infants may be detected by use of the specific strategies outlined in this article. We believe that this approach to testing, particularly in the framework of a national registry, will give paediatricians an opportunity to elucidate the true incidence, natural history and prognosis of an important, but perhaps neglected, disease of childhood.
1 Maternal hepatitis C virus (HCV) screening undertaken for pregnant women on methadone maintenance treatment, and those not on methadone treatment
 | |||||||||||||||
|
* P values represent differences between methadone and non-methadone groups. | |||||||||||||||
2 Follow-up appointment attendance for infants born to mothers who were on methadone maintenance treatment and were seropositive for hepatitis C virus*
 | |||||||||||||||
3 Rates of 18-month follow-up appointment attendance and rates and results of hepatitis C virus (HCV) screening tests, for infants born to HCV-seropositive mothers at a metropolitan birthing centre
 | |||||||||||||||
4 Hepatitis C virus (HCV) screening recommendations for pregnant women and their infants
We recommend that the development of a national HCV registry, similar to the existing National HIV Database,25 be considered. This would enable tracking of children with HCV infection, hence a reduction of the loss to follow-up over children’s lifetimes, and provide a greater understanding of the natural history and progression of HCV infection. It would also provide reliable data on which to base future recommendations.
We reinforce the Royal Australian and New Zealand College of Obstetricians and Gynaecologists recommendation that all pregnant women be screened for HCV infection. To achieve broader implementation of this recommendation, we propose that, at the first antenatal visit, blood be collected and tested for anti-HCV antibodies, and collected and stored for RNA testing. RNA testing should only proceed for patients who are HCV seropositive. This would address non-compliance with respect to RNA testing and minimise the costs of universal RNA testing.
In addition, we recommend that infants of HCV RNA-positive mothers be tested for HCV RNA at 6 weeks and 6 months of age, and tested for anti-HCV antibodies at 18 months of age. We justify our recommendation of testing at 6 weeks as infants are more likely to be followed up at an earlier appointment, and perinatal-acquired infection can be detected at this age. Testing at 6 months may capture data on clearance of neonatal infection — that is, identify infants with chronic or persistent infection.
Received 18 March 2009, accepted 7 September 2009
- Anthony J W Liu,*1
- Ethan I An,* 1
- Henry G Murray2
- Emma Tetstall1
- Marcel J Leroi3
- Ralph K H Nanan1
- 1 Discipline of Paediatrics, Sydney Medical School — Nepean, University of Sydney, Sydney, NSW.
- 2 Department of Obstetrics and Gynaecology, Nepean Hospital, Sydney West Area Health Service, Sydney, NSW.
- 3 Department of Pathology, Nepean Hospital, Sydney West Area Health Service, Sydney, NSW.
We thank Beth Bendall and Barbara Crowhurst for assistance with the data extraction.
None identified.
- 1. Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat 1999; 6: 35-47.
- 2. Razali K, Thein HH, Bell J, et al. Modelling the hepatitis C virus epidemic in Australia. Drug Alcohol Depend 2007; 91: 228-235.
- 3. Hallinan R, Byrne A, Amin J, Dore GJ. Hepatitis C virus prevalence and outcomes among injecting drug users on opioid replacement therapy. J Gastroenterol Hepatol 2005; 20: 1082-1086.
- 4. MacDonald MA, Wodak AD, Dolan KA, et al. Hepatitis C virus antibody prevalence among injecting drug users at selected needle and syringe programs in Australia, 1995–1997. Med J Aust 2000; 172: 57-61. <MJA full text>
- 5. Selvey LA, Denton M, Plant AJ. Incidence and prevalence of hepatitis C among clients of a Brisbane methadone clinic: factors influencing hepatitis C serostatus. Aust N Z J Public Health 1997; 21: 102-104.
- 6. Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345: 41-52.
- 7. Birnbaum AH, Shneider BL, Moy L. Hepatitis C in children. N Engl J Med 2000; 342: 290-291.
- 8. Spencer JD, Latt N, Beeby PJ, et al. Transmission of hepatitis C virus to infants of human immunodeficiency virus-negative intravenous drug-using mothers: rate of infection and assessment of risk factors for transmission. J Viral Hepat 1997; 4: 395-409.
- 9. Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronagative for HIV-1. BMJ 1998; 317: 437-441.
- 10. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. N Engl J Med 1994; 330: 744-750.
- 11. Alter MJ, Margolis HS, Krawczynski K, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med 1992; 327: 1899-1905.
- 12. Shakil AO, Conry-Cantilena C, Alter HJ, et al. Volunteer blood donors with antibody to hepatitis C virus: clinical, biochemical, virologic, and histologic features. The Hepatitis C Study Group. Ann Intern Med 1995; 123: 330-337.
- 13. NSW Department of Health. National clinical guidelines for the management of drug use during pregnancy, birth and the early development years of the newborn. Canberra: Commonwealth of Australia, 2006. http://www.health.nsw.gov.au/pubs/2006/pdf/ncg_druguse.pdf (accessed Jan 2009).
- 14. Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Hepatitis C. College statement 2007. http://www.ranzcog. edu.au/publications/statements/C-gen4.pdf (accessed Jan 2009).
- 15. Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia — data only, 2006. Canberra: ABS, 2006. (ABS Cat. No. 2033.0.55.001.) http://www.abs.gov.au/AUSSTATS/ abs@.nsf/allprimarymainfeatures/A1C03322C1AD9CF0CA2575DA00156075?opendocument#6 (accessed Oct 2009).
- 16. Australian Bureau of Statistics. National Regional Profile: Blacktown (C) (Local Government Area). Canberra: ABS, 2006. http://www.abs.gov.au/AUSSTATS/abs@.nsf/Latestproducts/LGA10750Population/People12002-2006?opendocument&tabname=Summary&prodno=LGA10750&issue=2002-2006 (accessed Mar 2009).
- 17. Australian Bureau of Statistics. National Regional Profile: Penrith (C) (Local Government Area). Canberra: ABS, 2006. http://www.abs. gov.au/AUSSTATS/abs@.nsf/Latestproducts/LGA16350Population/People12002-2006 ?opendocument&tabname=Summary& prodno=LGA16350&issue=2002-2006 (accessed Mar 2009).
- 18. Australian Bureau of Statistics. National Regional Profile: New South Wales. Canberra: ABS, 2006. http://www.abs.gov.au/AUSSTATS/abs@.nsf/Latestproducts/LGA1Population/People12002-2006?opendocument&tabname=Summary&prodno=LGA1&issue=2002-2006 (accessed Mar 2009).
- 19. Hardikar W, Elliott EJ, Jones CA. The silent infection: should we be testing for perinatal hepatitis C and, if so, how [editorial]? Med J Aust 2006; 184: 54-55. <MJA full text>
- 20. Alter MI, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999; 341: 556-562.
- 21. Conry-Cantilena C, Van Raden M, Gibble J, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med 1996; 334: 1691-1696.
- 22. Spencer JD, Tibbits D, Tippet C, et al. Review of antenatal testing policies and practice for HIV and hepatitis C infection. Aust N Z J Public Health 2003; 27: 614-619.
- 23. Tetstall E, Liu AJW, An E, et al. Pregnancy and neonatal characteristics of opioid-dependent Indigenous Australians: a rural and metropolitan comparison. Aust N Z J Obstet Gynaecol 2009; 49: 279-284.
- 24. Kaldor J, Jones CA, Elliott E, et al. Hepatitis C virus infection. In: Elliott EJ, Cronin P, Rose D, Zurynski Y, editors. Australian Paediatric Surveillance Unit surveillance report, 2002–2003. Sydney: APSU, 2005: 37-38.
- 25. National HIV/AIDS Strategy: revitalising Australia’s response 2005–2008. Canberra: Commonwealth of Australia, 2005.





Abstract
Objective: To describe the patterns of screening for hepatitis C virus (HCV) infection in methadone-maintained pregnant women and their infants.
Design, setting and patients: Retrospective review of medical records from one rural and two metropolitan hospitals in New South Wales for pregnant women on methadone maintenance treatment and infants born to these women between 1 January 2000 and 31 December 2006, as well as records for pregnant women who were not on methadone treatment.
Main outcome measures: Rates of anti-HCV antibody and HCV RNA testing for pregnant women and their infants, and ages at which infants attended follow-up appointments.
Results: Of 295 pregnant women on methadone maintenance treatment, 288 were tested for anti-HCV antibodies (98%), compared with 1995 of 9987 women who were not on methadone treatment (20%) (P < 0.001). Seropositive results were obtained for 243 women in the methadone group (84%) and 54 in the non-methadone group (3%) (P < 0.001), of whom 44 (18%) and 17 (31%), respectively, were subsequently tested for HCV RNA (P = 0.03). HCV RNA test results were positive for 31 (70%) and 10 (59%) seropositive women in the methadone and non-methadone groups, respectively (P = 0.39). Of infants of HCV-seropositive methadone-maintained mothers, 27% of those for whom we had follow-up attendance data received HCV screening, and one of these infants tested positive for anti-HCV antibodies and HCV RNA.
Conclusions: Screening for HCV infection in the high-risk population of pregnant women on methadone maintenance treatment and their infants is inadequate. This could lead to significant underdetection of active HCV infection in this high-risk population, and their infants. Current screening guidelines may therefore need to be revised.