Enteral tube feeding is a form of artificial nutrition that provides nutrients, hydration and medications on a long-term basis (> 1 month) directly into the gastrointestinal tract rather than via the mouth. It is predominantly used in older people with debilitating conditions (advanced head or neck tumours,1 motor neurone disease2 and stroke complicated by dysphagia3) who have a functioning and accessible gastrointestinal tract but an impaired swallowing mechanism. The most widely used method for enteral feeding involves placing a feeding tube directly into the stomach via percutaneous endoscopic gastrostomy (PEG),4 a minimally invasive, non-surgical procedure introduced in the 1980s.5 Despite its simplicity, enteral feeding remains controversial owing to uncertain benefits and considerable risks, and because it often involves patients without capacity and with unknown preferences within a complex ethical and legal framework.6
We obtained de-identified hospital admissions data linked to mortality data from the WA Data Linkage System. The study population included all patients 65 years or older who were admitted to a public or private hospital in Western Australia for an initial placement of a feeding tube, as defined by procedural codes of the International classification of diseases, injuries and causes of death, ninth revision, clinical modification (ICD-9-CM) (codes 43.1, 43.11, 43.19) and the International statistical classification of diseases and related health problems, 10th revision, Australian modification (ICD-10-AM) (codes 30481-00, 30483-00, 30375-00).7,8 A 5-year inpatient look-back period was used to confirm that patients identified by ICD-10-AM codes were undergoing these procedures for the first time. The data sources did not include additional clinical information, such as who was involved in the decision to place a GT, the severity of dementia, or the location to which the patient was discharged (home or residential aged care).
Indications for GT placement were determined using the primary diagnosis of the index (first) GT record, categorised according to ICD chapters. Additionally, six conditions of interest — cerebrovascular disease, dementia, cancer without metastases, cancer with metastases, motor neurone disease and Parkinson’s disease — were identified from all diagnosis fields for all hospital admissions in the 12-month period up to and including the index GT admission. The Charlson Comorbidity9 score was calculated similarly. Each member of the cohort was followed up for a maximum of 3 years from their index GT placement inpatient record.
Estimates of cumulative mortality by age (65–74, 75–84, 85 + years) and sex were calculated at 7 days, 30 days, 60 days, 180 days, 1 year and 3 years after GT placement, using the Kaplan–Meier method.10 Hazard ratios for the six conditions of interest were calculated using Cox proportional hazards regression, adjusting for age, sex, Charlson Comorbidity score, and year of GT placement. As we found that the Charlson Comorbidity score had little association with the log hazard ratio across scores 1 to 3, but was associated with a linear increase in the log hazard ratio for scores 4 or higher, we modelled the effect of the Charlson Comorbidity score as two linear splines. Similarly, age was fitted as a continuous variable as we found it was linearly associated with the log hazard ratio. The proportional hazards assumption was assessed by examining the Schoenfeld residuals for each covariate. Data were analysed using Stata release 10 (StataCorp, College Station, Tex, USA).
In WA, 2023 people aged 65 years or older underwent a GT placement for the first time between 1994 and 2004 (Box 1). Men tended to have their first GT placement at a younger age (median, 76.5 years; interquartile range [IQR], 71.5–82.3 years) than women (median, 79.3 years; IQR, 73.2–84.8 years). The number of initial GT placement procedures performed each year varied. In 1994, 83 were performed; in 1999, the annual number of procedures had increased to 252; and the number then declined by about a third to 173 in 2002.
On review of hospital admissions in the 12 months before the index admission, more than half of GT recipients (50.3%) had a known history of cerebrovascular disease. Other medical conditions identified are listed in Box 2. In people with dementia at the time of GT placement (11.2%), almost half also had cerebrovascular disease (47.6%) and 14.1% had Parkinson’s disease. Malnutrition at the time of the index admission was rare (1.7%).
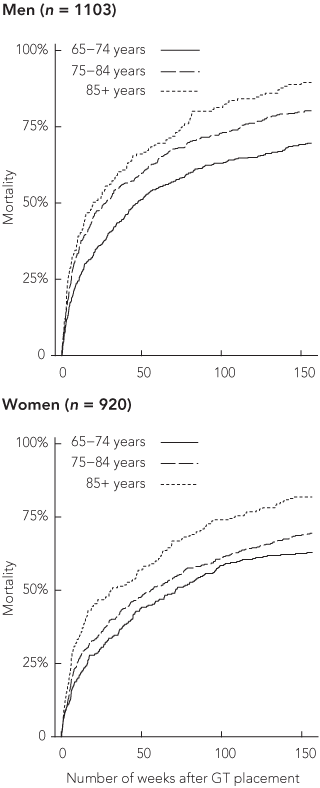
Mortality after GT placement was substantial. At 30 days, 16.6% of recipients had died (95% CI, 15.1%–18.3%), and the proportion of patients who had died increased to 26.3% by 60 days (95% CI, 24.4%–28.3%), 54.2% by 1 year (95% CI, 52.1%–56.4%), and 74.1% (95% CI, 72.1%–75.9%) by 3 years. Median survival was 53.3 weeks for women (95% CI, 45.1–63.4) and 34 weeks for men (95% CI, 29.1–40.3). The survival advantage of women compared with men was evident across all age groups (Box 3).
The risk of mortality varied among the six conditions of interest (Box 4), after adjusting for age, sex, other comorbidities (using the Charlson Comorbidity score) and year of GT placement. The risk of mortality was 81% higher in patients with motor neurone disease than those with other conditions. In cancer patients, the mortality risk was increased by 37% among those without metastases, and by 53% among those with metastases. The risk in patients with cerebrovascular disease was 13% lower than in other patients. In patients with dementia, the risk was 15% higher than in other patients, but the confidence interval around this estimate is wide, hence chance alone might account for this result. The risk in patients with Parkinson’s disease was not different to the risk in other patients. Overall, the mortality risk in women was 19% lower than in men, and each year of age was associated with a 4% increase in mortality risk. Even though the number of procedures performed during each year of the study varied considerably (Box 1), calendar year was not associated to any large degree with mortality risk. For Charlson Comorbidity scores below 4, there was no association between score and mortality risk, but for scores of 4 or more, each unit increase in the score was associated with a 4% increase in mortality risk.
Despite substantial variation in the numbers of index GT placement procedures performed each year in WA during the period 1994–2004, there was no evidence of a secular change in mortality. The variation was not an artefact of changes to the ICD coding of GTs, or a result of any initiatives introduced by the WA Department of Health. It is possible that the dramatic increase in GT placement procedures that occurred during the period 1997–1999 coincided with the introduction of the Aged Care Act 1997 (Cwlth), which was associated with an exhaustive accreditation process and little direction about the best practice for a palliative approach in residential aged care facilities. The decline in GT placements thereafter may be associated with publications such as the third edition of the Medical care of older persons in residential aged care facilities11 (published in 1999), which promoted explicit discussion and planning of end-of-life decisions with residents.
The prevalence of medical conditions differed markedly depending on whether estimates were derived from the primary diagnosis reported on the inpatient record for the index GT placement only, or also used additional data from co-diagnosis fields and a 12-month retrospective period. We argue that, using Australian inpatient data, the latter represents a superior approach, which results in greater disease-specific case ascertainment (eg, 28% v 50% for cerebrovascular disease). Our prevalence estimates for cerebrovascular disease and neurodegenerative disorders were considerably lower than those reported from a United States community-based study (28% v 40% and 8% v 35%, respectively),12 perhaps reflecting the different settings. Alternatively, these data may represent real differences in GT usage between WA and the US.
Mortality during the 12 months after GT placement was substantial. Our results are broadly comparable with pooled international mortality estimates, except for a noticeably lower 1-year mortality estimate in our study (54% v 62%).13 The reason for this difference is unclear. It might be explained by differences in the clinical features of the populations studied — for example, higher prevalence of neurodegenerative disorders overseas, and differences in disease duration or age of disease onset. It may also imply that Western Australian clinicians are more selective in the use of GTs.
The explanation for international differences is likely to be multifactorial, particularly as feeding tube practice is influenced by a range of complex legal, professional and ethical factors that are external to the patient’s clinical features. These include caregiver convenience, differences in referral patterns, financial incentives, organisational factors (size, location, staffing), ethnicity and other demographic characteristics.12,14-16 Uncertainty among physicians about the indications for GT placement17 and regulatory concerns, particularly about being sanctioned for “starvation” or “mistreatment”, may also promote international variation, including the use of feeding tubes in a manner that is inconsistent with medical advice.18
Few studies have examined mortality beyond the first 12 months,19 and we found that one in four GT recipients lived for up to 3 years. We found no evidence to support poorer survival after GT placement in older people with dementia, which is consistent with previously published data.16 Older people with cerebrovascular disease had significantly better survival in the 3 years after GT placement compared with all other GT recipients. In contrast, the survival of older people with metastatic cancer or motor neurone disease was substantially poorer. These findings show that enteral feeding may serve different purposes depending on the clinical features of the patient.4
GT-related complications were not uncommon. Rehospitalisation within 1 year for a GT replacement procedure, mechanical complications and incident pneumonitis occurred in 13%, 4% and 9% of patients, respectively. These are probably underestimates, as we only counted those patients who had complications that were severe enough to require hospitalisation. Other complications that may have been managed in an outpatient or community setting include peristomal infection; leakage; tube removal, displacement or migration; bleeding; gastric mucosal overgrowth or ulceration; metabolic and biochemical complications (eg, refeeding syndrome); gastrointestinal side effects; and microbial contamination and infection of feed.20,21
It is uncertain whether the use of GTs is accompanied by demonstrable health benefits, patient comfort and a satisfactory quality of life, or whether their use inadvertently interferes with the dying process and prolongs suffering.12 This issue is complicated by various medicolegal and ethical considerations concerning the use of feeding tubes. The Australian Medical Association has described artificial nutrition and hydration (ANH) as a life-sustaining treatment, and stated that the cessation or non-initiation of such measures is in accordance with good medical practice and does not constitute euthanasia or physician-assisted suicide.22 Also, competent adults have the legal right to accept or refuse any medical treatment, including ANH, and in WA and most other Australian states and territories, competent adults are able to complete advance health directives (“living wills”), including directives to withdraw or withhold life-sustaining measures such as ANH when they are no longer competent.23
1 Characteristics of older patients who underwent gastrostomy tube placement, Western Australia, 1994–2004
2 Medical conditions of older patients who underwent gastrostomy tube placement, Western Australia, 1994–2004*
3 Cumulative mortality of older men and women by age, after gastrostomy tube (GT) placement, Western Australia, 1994–2004
Received 2 September 2008, accepted 22 December 2008
- Janine Calver1
- Kieran A McCaul1
- Melinda Burmas2
- Barbara J Horner3
- Leon Flicker1
- 1 Western Australian Centre for Health and Ageing, University of Western Australia, Perth, WA.
- 2 Data Linkage WA, University of Western Australia, Perth, WA.
- 3 Centre for Research on Ageing, Curtin University of Technology, Perth, WA.
Data Linkage WA provided the data at no cost. We thank the staff of Data Linkage WA and the Information, Management and Reporting Directorate of the WA Department of Health for sharing their technical and clinical coding expertise. The study was partly funded by the Australian Government’s Dementia: a national health priority initiative.
None identified.
- 1. Scolapio JS, Spangler PR, Romano MM, et al. Prophylactic placement of gastrostomy feeding tubes before radiotherapy in patients with head and neck cancer: is it worthwhile? J Clin Gastroenterol 2001; 33: 215-217.
- 2. Leigh PN, Abrahams S, Al-Chalabi A, et al. The management of motor neurone disease. J Neurol Neurosurg Psychiatry 2003; 74 Suppl 4: iv32-iv47.
- 3. Allison MC, Morris AJ, Park RH, Mills PR. Percutaneous endoscopic gastrostomy tube feeding may improve outcome of late rehabilitation following stroke. J R Soc Med 1992; 85: 147-149.
- 4. Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA 1998; 279: 1973-1976.
- 5. Gauderer MW, Ponsky JL. A simplified technique for constructing a tube feeding gastrostomy. Surg Gynecol Obstet 1981; 152: 83-85.
- 6. Dharmarajan TS, Unnikrishnan D, Pitchumoni CS. Percutaneous endoscopic gastrostomy and outcome in dementia. Am J Gastroenterol 2001; 96: 2556-2563.
- 7. International classification of diseases, 9th revision, clinical modification: ICD-9-CM annotated. 7th ed. Ann Arbor, Mich: Commission on Professional and Hospital Activities, 1990.
- 8. International statistical classification of diseases and related health problems, 10th revision, Australian modification (ICD-10-AM). 4th ed. Sydney: National Centre for Classification in Health, 2004.
- 9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373-383.
- 10. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457-481.
- 11. Royal Australian College of General Practitioners. Medical care of older persons in residential aged care facilities — the silver book. 3rd ed. Melbourne: RACGP Communications Directorate, 1999.
- 12. Callahan CM, Haag KM, Weinberger M, et al. Outcomes of percutaneous endoscopic gastrostomy among older adults in a community setting. J Am Geriatr Soc 2000; 48: 1048-1054.
- 13. Mitchell SL, Tetroe JM. Survival after percutaneous endoscopic gastrostomy placement in older persons. J Gerontol A Biol Sci Med Sci 2000; 55: M735-M739.
- 14. Mitchell SL. Financial incentives for placing feeding tubes in nursing home residents with advanced dementia. J Am Geriatr Soc 2003; 51: 129-131.
- 15. Mitchell SL, Teno JM, Roy J, et al. Clinical and organizational factors associated with feeding tube use among nursing home residents with advanced cognitive impairment. JAMA 2003; 290: 73-80.
- 16. Higaki F, Yokota O, Ohishi M. Factors predictive of survival after percutaneous endoscopic gastrostomy in the elderly: is dementia really a risk factor? Am J Gastroenterol 2008; 103: 1011-1016.
- 17. Skelly RH. Are we using percutaneous endoscopic gastrostomy appropriately in the elderly? Curr Opin Clin Nutr Metab Care 2002; 5: 35-42.
- 18. Casarett D, Kapo J, Caplan A. Appropriate use of artificial nutrition and hydration — fundamental principles and recommendations. N Engl J Med 2005; 353: 2607-2612.
- 19. Golden A, Beber C, Weber R, et al. Long-term survival of elderly nursing home residents after percutaneous endoscopic gastrostomy for nutritional support. Nurs Home Med 1997; 5: 382-389.
- 20. Pearce CB, Duncan HD. Enteral feeding. Nasogastric, nasojejunal, percutaneous endoscopic gastrostomy, or jejunostomy: its indications and limitations. Postgrad Med J 2002; 78: 198-204.
- 21. DeLegge MH. Consensus statements regarding optimal management of home enteral nutrition (HEN) access. JPEN J Parenter Enteral Nutr 2006; 30 (1 Suppl): S39-S40.
- 22. Australian Medical Association. Position Statement on the role of the medical practitioner in end of life care. Canberra: AMA, 2007.
- 23. Parliamentary Library. Acts Amendment (Consent to Medical Treatment) Bill 2006. Perth: Parliament of Western Australia, 2008. http://www.parliament.wa.gov.au/parliament/bills.nsf/F6F3898DFC7C3104482571930042092D/$File/Bill149-2.pdf (accessed Feb 2009).





Abstract
Objective: To determine the number of older Western Australians who had a gastrostomy tube (GT) placement from 1994 to 2004, to describe their characteristics, and to examine outcomes after GT placement, including rehospitalisation for complications and survival.
Design and data sources: Secondary analysis of hospital (inpatient) data and linked mortality data from the WA Data Linkage System.
Main outcome measures: Patient characteristics (age, sex and morbidity profile); numbers of GT closures, replacements and complications within 1 year of GT placement; age- and sex-specific survival outcomes calculated at 7, 30, 60 and 180 days, and 1 and 3 years; and mortality hazard ratios calculated for six conditions of interest, identified using all available diagnosis information on the inpatient record.
Results: In Western Australia, 2023 people aged 65 years or older underwent a GT placement for the first time during the period 1994–2004, half of whom had a known history of cerebrovascular disease (50.3%). Rehospitalisation within 1 year for a GT replacement procedure, mechanical complications and incident pneumonitis occurred in 13%, 4% and 9% of patients, respectively. More than half of the patients who underwent a GT placement died within 1 year. Survival outcomes were poorest for patients with motor neurone disease and metastatic cancer.
Conclusion: To better understand this complex area of health care, questions regarding decision making — by patients, families, physicians, hospitals and other caring organisations — about GT placement and maintenance need to be addressed.