A 55-year-old woman who was treated with long-term, high-dose clozapine for schizophrenia presented with bilateral decreased visual acuity. She had pigmentary changes affecting the cornea and the retina, as well as stellate cataract. Chlorpromazine use is known to produce similar changes, but this is the first report to our knowledge of pigmentation associated with clozapine use. (MJA 2009; 190: 210-211)
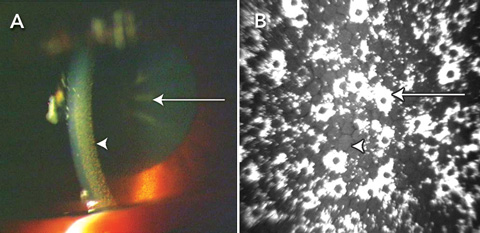
The patient’s best corrected visual acuity was 6/9 in the right eye and 6/60 in the left. Bilateral pigmented deposits were present in the corneal endothelium, and these were most prevalent in the interpalpebral fissure. On dilation, bilateral pigment dusting of the anterior portion of the lens capsule with central stellate opacity was evident (Box, A), and posterior subcapsular and nuclear sclerotic cataract was noted in both eyes. Retinal changes included a right epiretinal membrane and bilateral pigmentary retinopathy. Macular atrophy was present in both eyes and affected fixation on the left. The patient’s skin was brown, particularly in sun-exposed areas including her face, neck and hands.
Confocal microscopy of the corneas showed diffuse, highly reflective, irregular honeycomb-shaped deposits on the endothelium (Box, B) and small granular deposits on the posterior stromal layer. Morphology of the endothelium visible between the deposits was normal. Optical coherence tomography confirmed atrophy of the neuroretina, greater in the left eye than the right. Electroretinography showed reduced cone function, indicated by reduced amplitude and latency in the cone response.
Pigment deposits in the cornea, lens and skin are well documented complications of long-term phenothiazine antipsychotic therapy.1-3 Our patient had ocular changes that were possibly side effects of chronic high-dose clozapine use. The changes were similar to the side effects of phenothiazines, and they were demonstrated by confocal microscopy, optical coherence tomography and electroretinography.1-4 Medication history taken from the patient, as well as a collaborative medication history supplied by the patient’s psychiatric team, revealed no evidence of prior phenothiazine use.
Clozapine therapy is usually commenced at a dose of 25 mg daily, and titrated up to 300–600 mg daily for therapeutic effect. Doses of up to 900 mg can be used for treatment-resistant cases.5 Clozapine is recommended as a substitute for patients who have experienced pigmentation secondary to chlorpromazine use — clinical signs of pigmentation are expected to resolve after a period of chlorpromazine abstinence.6
The aetiology of phenothiazine-related ocular side effects has not been determined. It has been postulated that photosensitisation of tissue proteins occurs in areas with increased sun exposure after accumulation of the drug in these tissues.7 Alternatively, phenothiazines may interact with melanin in the choriocapillaris and retinal pigment in the epithelium, which may induce damage to the photoreceptors. Altered dopaminergic regulation of melatonin is suspected to increase susceptibility of photoreceptors to damage by light.7
In our patient, clozapine may have produced similar side effects to the phenothiazines, as it also acts on dopamine receptors. The dopaminergic system of the retina may respond to accumulation of clozapine and phenothiazines in a similar manner to the nigrostriatal dopaminergic system.8 None of the other medications the patient was taking — namely lithium, omeprazole or thyroxine — are known to cause skin or ocular pigmentation.9,10
- 1. Petrohelos MA, Ticoulis D. Ocular complications of chlorpromazine therapy. Ophthalmologica 1969; 159: 31-38.
- 2. Bock E, Swain J. Ophthalmologic findings in patients on long-term chlorpromazine therapy. Am J Ophthalmol 1963; 56: 808-810.
- 3. Greiner AC, Berry K. Skin pigmentation and corneal and lens opacities with prolonged chlorpromazine therapy. Can Med Assoc J 1964; 90: 663-665.
- 4. Phua YS, Patel DV, McGhee CN. In vivo confocal microstructural analysis of corneal endothelial changes in a patient on long-term chlorpromazine therapy. Graefes Arch Clin Exp Ophthalmol 2005; 243: 721-723.
- 5. Novartis Pharmaceuticals Australia Pty Ltd. Product information (abbreviated): Clozaril (clozapine). 23 Mar 2007.
- 6. Lal S, Lal S. Chlorpromazine-induced cutaneous pigmentation — effect of replacement with clozapine. J Psychiatry Neurosci 2000; 25: 281.
- 7. Deluise VP, Flynn JT. Asymmetric anterior segment changes induced by chlorpromazine. Ann Ophthalmol 1981; 13: 953-955.
- 8. Cohen J, Iuvone PM, Neff NH. Neuroleptic drugs activate tyrosine hydroxylase of retinal amacrine cells. J Pharmacol Exp Ther 1981; 218: 390-394.
- 9. Lam RW, Allain S, Sullivan K, et al. Effects of chronic lithium treatment on retinal electrophysiologic function. Biol Psychiatry 1997; 41: 737-742.
- 10. García Rodríguez LA, Mannino S, Wallander MA, et al. A cohort study of the ocular safety of anti-ulcer drugs. Br J Clin Pharmacol 1996; 42: 213-216.





None identified.