Chronic hepatitis B (HBV) and hepatitis C (HCV) infections cause significant morbidity and mortality through sequelae such as liver fibrosis, cirrhosis and hepatocellular carcinoma. Together, they are the most common indication for adult liver transplantation in Australia.1 Antiviral therapy is the standard care for adult patients with HCV infection, preventing progression to end-stage disease, and is used in patients with HBV who meet specific criteria.2 Treatment of children can be as effective as in adults, particularly for HCV.3 However, antiviral therapy for HCV is not currently approved in the Australian Pharmaceutical Benefits Scheme for those under 18 years of age, thus restricting access to the medications. Chronic HBV or HCV infection in children is usually asymptomatic and may remain undetected, although significant liver disease can occur.4,5
Characteristics of the 79 patients with HBV infection are summarised in Box 1. Fifty-one patients were male, and the mean age at referral was 9.1 years (range, 1–17 years). Most were refugees born in Africa or migrants from Asia. Half of the patients born in Australia or New Zealand had parents who were both born overseas. Vertical transmission (38/79) was more common than horizontal transmission (10/79), but the mode of transmission could not be determined for 31 patients. Seven patients developed HBV infection despite a history of receiving neonatal vaccination and hepatitis B immunoglobulin at birth. Apart from hepatomegaly in one patient and splenomegaly in another, all other patients had normal results of a gastroenterological examination.
There was only one patient for whom eAg/eAb status was not known. Most (56/79) were in the immune tolerant eAg-positive/eAb-negative phase of infection (Box 1). Most of these patients had high viral loads (median, 1.7 × 106 IU/mL), with either normal or slightly raised alanine aminotransferase (ALT) levels. Eighteen patients had undergone eAb seroconversion and had cleared eAg. These patients had lower viral loads and ALT levels, consistent with suppressed viral replication (data not shown). Four patients were positive for both eAg and eAb at presentation. One patient out of the 56 who were initially eAg-positive/eAb-negative had spontaneous seroconversion and became eAg-negative with a low viral load during follow-up. No patients were eAg-negative/eAb-positive with a high viral load.
Liver biopsy was performed on eight patients during the study period because of persistent elevation in transaminase levels, to assess for the degree of inflammation and fibrosis, and for consideration of possible therapy. Two patients had bridging fibrosis evident on biopsy, but none had cirrhosis. Three had mild and two had moderate necroinflammatory activity (by Ishak grading).6
Characteristics of the 29 patients with HCV infection are summarised in Box 2. Again, there was a male predominance, but most patients were born in Australia. The mean age at referral was 7.8 years (range, 1–14 years). All patients were asymptomatic and none had clinical signs of chronic liver disease at referral.
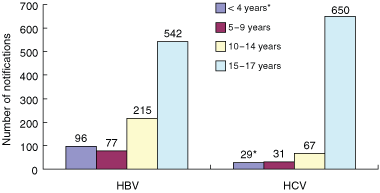
Notifications to NSW Health from 2000 to 2007 of children with HBV and HCV infection are shown in Box 3. During this period, there were 930 HBV notifications for children aged < 18 years and 777 HCV notifications in children aged between 18 months and 18 years. While the 15–17-years age group had the most notifications, 388 children aged less than 15 years with HBV and 127 children aged between 18 months and 15 years with HCV were identified during the 8-year period.
Most of the children with HBV infection were either refugees or migrants, or children of refugees or migrants. As refugees are often screened after arrival in Australia, there will be an ascertainment bias in this group. The mode of transmission was unclear for almost 40% of children. Almost half of the Australian-born children had apparently received neonatal prophylaxis with both vaccination and immunoglobulin. These measures have an efficacy of between 70% and 100% in preventing vertical transmission from an eAg-positive mother, depending on her viral load.7 Our patients in whom this apparently failed underline the need for close follow-up of infants who receive prophylaxis. While most had biochemical evidence of hepatitis, advanced liver disease was uncommon and very few had received treatment.
Age at infection is an important factor in the natural history — children infected vertically or within the first 5 years of life have higher rates of chronic infection (25%–30%) than those infected later in life (5%–10%).8 Similarly, eAg seroconversion rates vary from 2%–5% per year in young, vertically infected children9 to up to 70%–80% of horizontally infected older children by age 20.10 The dominant mode of transmission to children varies by geographical location, with higher rates of horizontal infection in Africa than in eastern Asia, where vertical transmission is most common.11 These differences may be explained by different HBV genotypes.12 The mix of geographical origins and differing modes of infection in our group of patients may thus result in varied natural histories.
Cirrhosis occurs in around 20% of adults with chronic HBV infection over a 20-year period13 and is a precursor to hepatocellular carcinoma. About 4% of horizontally acquired HBV infections in childhood progressed to cirrhosis in one study spanning 29 years,14 although half of these patients developed hepatocellular carcinoma. In Taiwan, where vertical transmission is common, the annual incidence of cirrhosis in young eAg-positive adults was found to be 2.4%.15
Development of progressive liver fibrosis is generally associated with prolonged elevation of ALT levels,16 but can develop in those with normal ALT levels.5 Those who have seroconverted can also experience reactivation with either eAg-positive or eAg-negative chronic hepatitis, resuming the risks of increased infectivity and progression of liver disease.17 These features of HBV infection mandate long-term follow-up for disease progression, with biopsies and therapy if necessary.
Treatment of HBV in children is focused on those who are shown to be developing significant, irreversible liver damage on biopsy.3 The decision to treat is difficult because of uncertainty about the required length of therapy — when it is ceased, viral loads often rebound. Also, the higher spontaneous eAg seroconversion rate in children, the risk of resistant mutants arising, side effects of medications, and the relatively long lead time from infection to the development of serious liver disease make treatment decisions complicated. Based on paediatric trials, the best evidence for efficacy exists for interferon α-2b,18 lamivudine,19 and, more recently, adefovir.20 However, there is also evidence that there may be less response to antiviral therapy in younger children.20
In contrast to the patients with HBV, those with HCV infection were mostly Australian-born and acquired infection vertically, typically from mothers with a history of injecting drug use. Few had evidence of significant liver disease, although about half of the patients who had HCV genotyping had genotypes 2 or 3, which have a “cure rate” of greater than 80% with antiviral therapy.3
The natural history of paediatric HCV infection is still being elucidated. Around 10%–20% of adults progress to cirrhosis within 20 years of infection.21 One paediatric cohort study suggests that 8% will clear HCV spontaneously within 10 years, and 1.8% will progress to cirrhosis over the same period.22 Clearance was more likely with vertically infected children and those with genotype 3, while cirrhosis was more likely with genotype 1 or persistently elevated ALT levels.22 Rates of histological bridging fibrosis (a precursor to cirrhosis) vary from 4% to 22% in studies, probably related to different genotypes and modes of acquisition.4,23 It is likely that many of these patients will progress to cirrhosis in adulthood; however, decompensated cirrhosis has been described in children.22
The standard first-line treatment for HCV is pegylated interferon α combined with ribavirin, which achieves a sustained virological response (undetectable HCV RNA 24 weeks after stopping therapy) in over 40% of adults with genotype 1 and around 80% with genotypes 2 or 3.24 Similar results have been achieved in paediatric trials.25 Treatment of our group of patients could eliminate chronic HCV infection in a significant proportion, sparing them considerable morbidity, and possibly mortality, later in life. There would also be a health expenditure saving, as well as psychosocial benefits for the individual who would no longer be burdened by the social stigma of the disease. To date, none of our patients have been treated because of a lack of access to medication and insufficient clinic resources to enable treatment.
Received 16 October 2008, accepted 4 March 2009
- Scott Nightingale1
- Michael O Stormon1
- Andrew S Day2,3
- Murray T Webber4,5
- Kate A Ward6
- Edward V O’Loughlin1
- 1 Department of Gastroenterology, The Children’s Hospital at Westmead, Sydney, NSW.
- 2 Department of Gastroenterology, Sydney Children’s Hospital, Sydney, NSW.
- 3 School of Women’s and Children’s Health, University of New South Wales, Sydney, NSW.
- 4 Hunter New England Area Health Service, Newcastle, NSW.
- 5 School of Medicine and Public Health, University of Newcastle, Newcastle, NSW.
- 6 Communicable Diseases Branch, NSW Health, Sydney, NSW.
We wish to thank Professor David Isaacs and Dr Nick Wood (Children’s Hospital at Westmead) and Dr Asha Bowen (Sydney Children’s Hospital) for contributing data on patients from their clinics for analysis.
None identified.
- 1. Australian National Liver Transplantation Unit. Liver transplantation at Australian National Liver Transplantation Unit: data to 31 December 2008. Sydney: ANLTU, 2009. http://www.sswahs.nsw. gov.au/Gastro/LiverTransplant/pdf/Rpah2008.pdf (accessed May 2009).
- 2. Liaw YF, Leung N, Guan R, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int 2005; 25: 472-489.
- 3. Elisofon SA, Jonas MM. Hepatitis B and C in children: current treatment and future strategies. Clin Liver Dis 2006; 10: 133-148.
- 4. Goodman ZD, Makhlouf HR, Liu L, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology 2008; 47: 836-843.
- 5. Kumar M, Sarin SK, Hissar S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology 2008; 134: 1376-1384.
- 6. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696-699.
- 7. del Canho R, Grosheide PM, Mazel JA, et al. Ten-year neonatal hepatitis B vaccination program, The Netherlands, 1982–1992: protective efficacy and long-term immunogenicity. Vaccine 1997; 15: 1624-1630.
- 8. McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151: 599-603.
- 9. Hsu HY, Chang MH, Chen DS, et al. Baseline seroepidemiology of hepatitis B virus infection in children in Taipei, 1984: a study just before mass hepatitis B vaccination program in Taiwan. J Med Virol 1986; 18: 301-307.
- 10. Bortolotti F, Jara P, Crivellaro C, et al. Outcome of chronic hepatitis B in Caucasian children during a 20-year observation period. J Hepatol 1998; 29: 184-190.
- 11. Kew MC. Progress towards the comprehensive control of hepatitis B in Africa: a view from South Africa. Gut 1996; 38 Suppl 2: S31-S36.
- 12. Livingston SE, Simonetti JP, Bulkow LR, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology 2007; 133: 1452-1457.
- 13. Ganem D, Prince AM. Hepatitis B virus infection — natural history and clinical consequences. N Engl J Med 2004; 350: 1118-1129.
- 14. Bortolotti F, Guido M, Bartolacci S, et al. Chronic hepatitis B in children after e antigen seroclearance: final report of a 29-year longitudinal study. Hepatology 2006; 43: 556-562.
- 15. Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology 1988; 8: 493-496.
- 16. Godra A, Perez-Atayde AR, Jonas MM. Histologic features of chronic hepatitis B in children [abstract]. Hepatology 2005; 42 Suppl 1: 478A-479A.
- 17. Ni YH, Chang MH, Chen PJ, et al. Viremia profiles in children with chronic hepatitis B virus infection and spontaneous e antigen seroconversion. Gastroenterology 2007; 132: 2340-2345.
- 18. Sokal EM, Conjeevaram HS, Roberts EA, et al. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology 1998; 114: 988-995.
- 19. Jonas MM, Mizerski J, Badia IB, et al. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med 2002; 346: 1706-1713.
- 20. Jonas MM, Kelly D, Pollack H, et al. Safety, efficacy, and pharmacokinetics of adefovir dipivoxil in children and adolescents (age 2 to <18 years) with chronic hepatitis B. Hepatology 2008; 47: 1863-1871.
- 21. Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001; 34: 809-816.
- 22. Bortolotti F, Verucchi G, Camma C, et al. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology 2008; 134: 1900-1907.
- 23. Badizadegan K, Jonas MM, Ott MJ, et al. Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology 1998; 28: 1416-1423.
- 24. Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology 2006; 130: 231-264.
- 25. Wirth S, Pieper-Boustani H, Lang T, et al. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology 2005; 41: 1013-1018.





Abstract
Objective: To characterise epidemiological, clinical and laboratory features of children in New South Wales with chronic hepatitis B (HBV) or C (HCV) infections.
Design and setting: Retrospective record review of epidemiological, clinical, laboratory, liver biopsy and treatment data for children (aged < 18 years) referred to tertiary referral paediatric and refugee clinics in NSW with chronic HBV or HCV during 2000–2007; and comparison with NSW Health notification data for the same period.
Main outcome measures: Numbers and characteristics of referred children with HBV and HCV, and notifications to NSW Health.
Results: During 2000–2007, 79 children with chronic HBV and 29 with HCV infection were referred to specialist clinics, while 930 children with HBV and 777 with HCV infection were reported to NSW Health. Most of the referred children with HBV were born overseas, while most with HCV were born in Australia to mothers with a history of intravenous drug use. Of the 79 HBV-infected children, 56 were e-antigen positive. Most HCV-infected children (23/29) had alanine aminotransferase levels ≤ 2 times the upper limit of normal, and more than half of those who had genotype determined had type 2 or 3. Fibrosis was evident in liver biopsies performed for both HBV and HCV.
Conclusions: Although advanced liver disease was uncommon in children referred with HBV or HCV infection, a large number of infected children in NSW were not referred for specialist medical care, indicating that opportunities to intervene early in the natural history of these infections, particularly HCV, are being missed.