An otherwise healthy 28-year-old man had a cardiac arrest after a day of motocross racing. He had consumed excessive amounts of a caffeinated “energy drink” throughout the day. We postulate that a combination of excessive ingestion of caffeine- and taurine-containing energy drinks and strenuous physical activity can produce myocardial ischaemia by inducing coronary vasospasm.
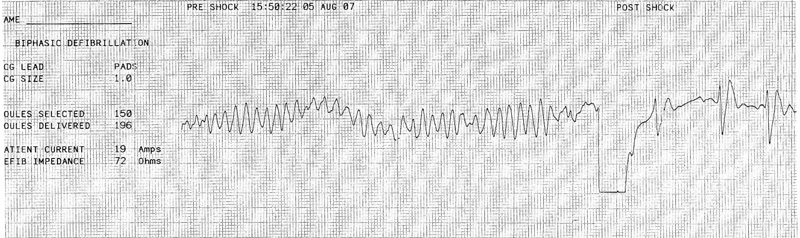
The patient’s initial cardiac rhythm was recorded as ventricular fibrillation (Box 1). He was restored to sinus rhythm after receiving two 150 J biphasic direct-current shocks. Adrenaline 1 mg and atropine 1 mg were both given as adjuvants. He was intubated by paramedics and transported to hospital.
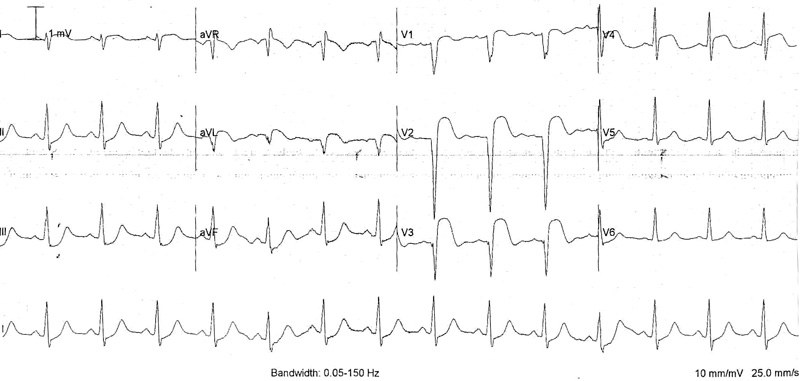
The patient was transferred to a tertiary referral centre for cardiac catheterisation. On arrival there, an ECG showed evolving ischaemic changes across the anterolateral leads (Box 2). A troponin I peak level of 12.2 mmol/L was measured; his potassium level had normalised at 4.0 mmol/L. Echocardiography showed mild left ventricular enlargement and low-normal systolic function with a hypokinetic anteroseptal segment.
Although sudden cardiac death is an uncommon occurrence in people under the age of 40 years, when it does happen it is most often associated with the presence of structural heart disease, most frequently premature coronary atherosclerosis. Other common associations are hypertrophic obstructive cardiomyopathy and myocarditis.1,2 However, autopsy review studies have found that some 10%–12% of subjects in this age group have no obvious cardiac abnormalities on postmortem examination.1,2 Of identified causes in this group, many are familial sudden cardiac deaths or disorders of conduction, such as Wolff–Parkinson–White syndrome.3
The role of illicit stimulants, especially cocaine, in causing coronary vasospasm in young people is well established.4 However, this patient denied cocaine use and returned a negative result on his drug test, making this an unlikely cause.
The energy drink consumed by our patient contains 80 mg of caffeine (equivalent to one cup of espresso) per can. He drank seven or eight cans within 7 hours — up to 640 mg of caffeine in total. The drink also contains high doses of taurine (an amino acid) and glucuronolactone (a glucose metabolite), neither of which are considered to have significant toxicity, although there is a paucity of data.5,6
Caffeine is a naturally occurring xanthine derivative related to theophylline; it has a number of potential pharmacological actions on the cardiovascular system. Its primary mechanism of action is thought to be through competitive inhibition of adenosine receptors.7 It also induces catecholamine release, and causes a rise in intracellular calcium in myocytes through release of calcium from the sarcoplasmic reticulum, leading variably to smooth muscle contraction and relaxation.8-10
The role of caffeine in triggering arrhythmia is well established.8 There have been a number of case reports on hospitalisations or deaths due to caffeine toxicity, although the mechanism usually seems to be tachyarrhythmia and involves far higher doses than in this case.11,12 The median lethal dose in rats is 200–400 mg/kg.13 A 1997 case report described a young woman who suffered a myocardial infarction due to caffeine toxicity; however, this involved an oral dose of 20 g.14
In-vitro studies have shown that taurine has an inotropic effect on cardiac muscle similar to that of caffeine, and potentiates caffeine-induced muscle contracture. Few taurine toxicity studies have been performed, and there are insufficient data to suggest what an unsafe level of taurine consumption might be, if any.9,14
Both taurine and caffeine have been shown in vitro to have physiological effects on intracellular calcium concentration within vascular smooth muscle, and they could conceivably induce coronary vasospasm. In-vivo studies have demonstrated a capacity for caffeine to decrease myocardial blood flow during exercise.15 We postulate that, in physiologically predisposed individuals, a combination of excessive ingestion of caffeine- and taurine-containing energy drinks and strenuous physical activity can induce myocardial ischaemia by coronary vasospasm, with potentially fatal results.
Caffeine has been removed from the list of prohibited substances in sport but remains on a monitoring program run by the World Anti-Doping Agency.16 Anecdotal reports suggest that the many caffeinated energy drinks now on the market are widely used by amateur and professional athletes to enhance their performance. We are concerned that a combination of exercise and the caffeine contained in these drinks may have the potential to trigger serious cardiovascular events.
- 1. Drory Y, Turetz Y, Hiss Y, et al. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol 1991; 68: 1388-1392.
- 2. Eckart RE, Scoville SL, Campbell CL, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med 2004; 141: 829-834.
- 3. Wever EF, Robles de Medina EO. Sudden death in patients without structural heart disease. J Am Coll Cardiol 2004; 43: 1137-1144.
- 4. Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med 2001; 345: 351-358.
- 5. CBC News. In the can: Red Bull’s ingredients dissected. http://www.cbc.ca/consumers/market/files/health/redbull/ingredients.html (accessed Jan 2008).
- 6. Australia New Zealand Food Authority. Inquiry report. Application A394: formulated caffeinated beverages. 8 August 2001. http://www.foodstandards.gov.au/_srcfiles/A394_(full)_report.pdf (accessed Jan 2008).
- 7. Haller CA. Caffeine. In: Olson KR, editor. Poisoning and drug overdose. International ed. The McGraw-Hill Companies, 2007.
- 8. Mehta A, Jain AC, Mehta MC, Billie M. Caffeine and cardiac arrhythmias. An experimental study in dogs with review of literature. Acta Cardiol 1997; 52: 273-283.
- 9. Steele DS, Smith GL, Miller DJ. The effects of taurine on Ca2+ uptake by the sarcoplasmic reticulum and Ca2+ sensitivity of chemically skinned rat heart. J Physiol 1990; 422: 499-511.
- 10. Watanabe C, Yamamoto H, Hirano K, et al. Mechanisms of caffeine-induced contraction and relaxation of rat aortic smooth muscle. J Physiol 1992; 456: 193-213.
- 11. Holmgren P, Nordén-Pettersson L, Ahlner J. Caffeine fatalities — four case reports. Forensic Sci Int 2004; 139: 71-73.
- 12. Cannon ME, Cooke CT, McCarthy JS. Caffeine-induced cardiac arrhythmia: an unrecognised danger of healthfood products. Med J Aust 2001; 174: 520-521. <MJA full text>
- 13. Organisation for Economic Co-operation and Development. SIDS [Screening Information Data Set] initial assessment profile: caffeine. Paris: OECD, Mar 2002. http://www.chemicals.moew.government.bg/chemical/site/File/registers/profile/58082p.pdf (accessed Jan 2008).
- 14. Forman J, Aizer A, Young CR. Myocardial infarction resulting from caffeine overdose in an anorectic woman. Ann Emerg Med 1997; 29: 178-180.
- 15. Baum M, Weiss M. The influence of a taurine containing drink on cardiac parameters before and after exercise measured by echocardiography. Amino Acids 2001; 20: 75-82.
- 16. World Anti-Doping Agency. The 2008 Monitoring Program. http://www.wada-ama.org/rtecontent/document/Monitoring_Program_2008_En.pdf (accessed Oct 2008).





We acknowledge and thank Dr Malcolm Barlow at John Hunter Hospital, Newcastle, and Professor Terry Campbell at St Vincent’s Hospital, Sydney, for their input in preparing this case report for publication.
None identified.