In Australia, prostate cancer is the most commonly diagnosed cancer after non-melanocytic skin cancer and the second most common cause of cancer death in men.1 Risk factors for prostate cancer include age, race and family history.
Population-wide screening for prostate cancer remains controversial.2 Since the prostate-specific antigen (PSA) test was listed on Australia’s Medicare Benefits Schedule (MBS) in 1988, it has become widely used in the work-up of lower urinary tract symptoms and as a de facto screening test for prostate cancer.3 Recognising deficiencies in the test’s characteristics and the uncertainty regarding optimal treatment for localised disease,4 relevant Australian bodies, including the Australian Government Department of Health and Ageing,5 do not recommend population-wide screening. However, the Urological Society of Australia and New Zealand recommends that healthy men aged 50–70 years be screened after giving appropriately informed consent.6
Data on all PSA tests in NSW residents reimbursed by Medicare between 1989 and 1997 were obtained from the then Commonwealth Department of Health and Family Services. Data on PSA tests reimbursed by Medicare between 1994 and 2006 were obtained from Medicare Australia.7 From May 2001, two classes of PSA test were distinguished in the MBS: “1 of this item in a 12 month period” (effectively screening); and “monitoring of previously diagnosed prostatic disease” or “quantitation of 2 or more fractions of PSA and any derived index ... in the followup of a PSA result which lies in the equivocal range of the particular method of assay”.8 Unless otherwise stated, our results refer to all PSA tests. Medicare data probably under-enumerate all PSA tests, as they do not include tests done in public hospitals.
Joinpoint regression finds the optimal points to identify changes in trends in rates. We used the Joinpoint Regression Program (version 3.0; National Cancer Institute, Bethesda, Md, USA)9 on age-specific rates, age-standardised incidence rates by spread of disease at diagnosis, and mortality rates by age groups to identify changes in trends.
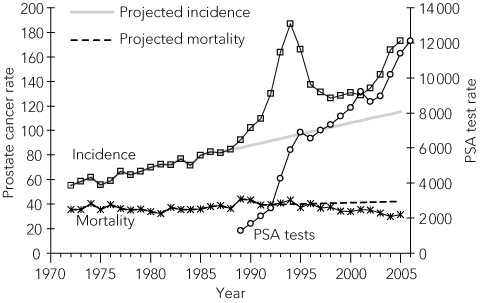
The age-standardised rate of PSA testing in NSW increased from 1284 per 100 000 men in 1989 to an initial peak of 6908 per 100 000 men in 1995 and then, after slight drops in 1996 and 2002, to a rate of 12 119 per 100 000 men in 2006 (Box 1). The annual number of PSA tests more than doubled from 184 350 in 1996 to 433 187 in 2006. The increase in test rate over this period averaged 6.2% annually. Based on the MBS classification of tests from 2001 to 2006, more than half (54%) were determined to be screening tests.
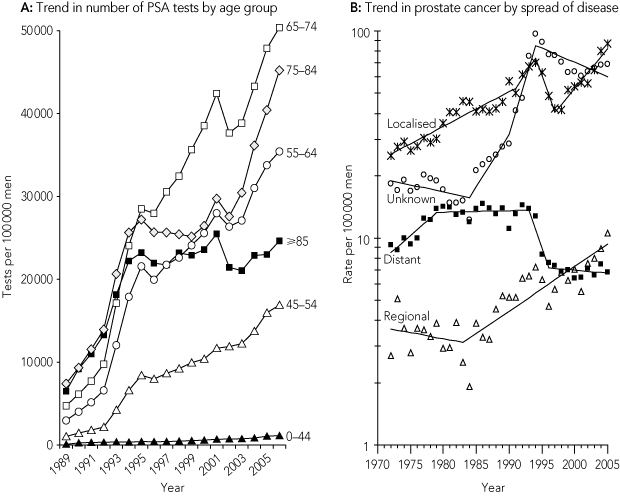
Total testing rates increased with age to 504 per 1000 in men aged 65–74 years in 2006 (Box 2, A). The highest rate of PSA testing for monitoring or follow-up occurred at older ages (75–84 years) (data not shown).
In 2005, 5950 men in NSW were diagnosed with prostate cancer. The age-standardised incidence of prostate cancer rose in 1989 from its long-term gentle upward trend and peaked in 1994 at 187 cases per 100 000 men, more than double the rate in 1989. It then fell by 10% per year to a minimum of 126 per 100 000 men in 1998, remained flat to 2001, and increased again by 4.9% per year to 2005 (Box 1).
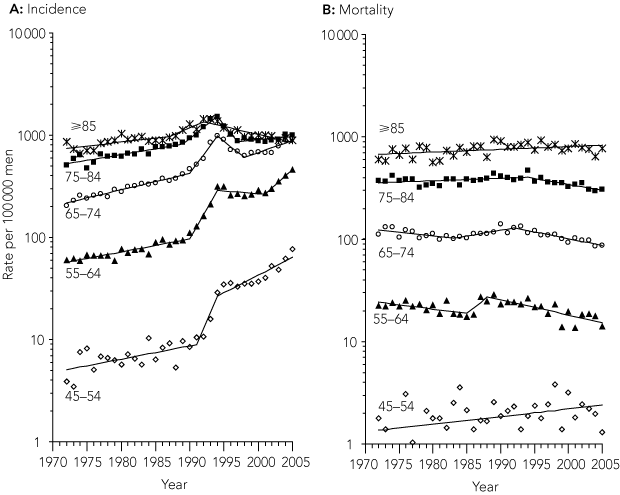
The relative increase in incidence rates was greatest in younger men: rates in those aged 45–54 years increased more than sevenfold from 7.5 per 100 000 men in 1982–1986 to 60.0 per 100 000 men in 2002–2005 (Box 3, A). Increases were first evident in 1988 in the oldest men, then in men aged 45–74 in 1991, which largely mirrored the initial uptake of PSA testing by age group (Box 2, A), with more tests occurring in older age groups between 1989 and 1993. Coinciding with PSA testing becoming available, the median age at which men were diagnosed with prostate cancer fell from 72 years in 1982–1986 to 69 years in 2005.
Trends in the age-standardised incidence of prostate cancer by CCR categories of recorded spread of disease at diagnosis are shown in Box 2, B. This analysis is limited by the high proportion with unknown spread at diagnosis, with 42% recorded as unknown in 2002–2005 compared with recorded figures of 47% with localised disease, 6% with regional spread and 4% with distant spread. The trends in incidence of recorded localised and unknown spread generally followed the trends in overall incidence, with an initial rise to the mid 1990s, then a fall to the late 1990s, followed by another increase. Systematic changes to CCR recording of unknown and localised spreads of disease during the 1990s may have affected these categories. The incidence of recorded distant spread, having been steady in the 1980s, fell from 13.0 per 100 000 men and 13.2% of newly diagnosed cases in 1987–1991 to 7.0 per 100 000 men and 4.2% of cases in 2002–2005.
There were 980 deaths from prostate cancer in 2005. The age-standardised mortality rate increased by 3.6% per year between 1984 and 1990 and then fell by 2.0% per year to 2005 (Box 1).
Projected incidence of prostate cancer (Box 1) remained substantially below the observed incidence from 1989 to 2005. We estimate that 19 602 extra men were diagnosed with prostate cancer, or about 43% more cases than were expected.
There was an excess of observed cases over projected cases in each year after 1989 in men aged 55–74 years. In men aged ≥ 75 years, an initial annual excess of observed cases became a deficit from 1997 onwards. There was no excess of cases in men younger than 45 years (data not shown). PSA testing below this age was uncommon (Box 2, A).
Observed mortality first fell below the projected trend in 1995 (Box 1). There were 1780 fewer deaths than expected during the period 1991–2005. The biggest deficit in observed deaths was in men aged 75–84 years. The deficit was smaller with each younger age group and negligible in men under 55 years of age. Mortality rates by age group are shown in Box 3, B.
Similar spikes in prostate cancer incidence following the introduction of PSA testing have been observed in other countries.10 A second increase around 2002, evident elsewhere in Australia,11 is probably a result of changes in diagnostic procedures. The detection of clinically significant cancers in up to 22% of men with PSA levels of 2.6–4.0 ng/mL12-14 led to a lowering of the investigation threshold for some clinicians from the previous level of 4.0 ng/mL.15,16 Data from the largest private pathology practice in NSW also show the proportion of biopsies obtaining more than seven cores increased from 11% in 1997 to 60% in 2007 (Warick Delprado, Douglass Hanly Moir Pathology, Sydney, NSW, personal communication). Increasing the number of cores to 10 or 11 can increase prostate cancer diagnosis by up to 31%.15,16 Additionally, the number of prostate biopsies in NSW increased by 59% between 2000 and 2004.7
The sustained decline in distant-stage disease to 2005 is consistent with a stage shift. In the United States, an average annual fall of 17.9% in distant-stage cancers occurred between 1991 and 1995.17 Falls have been reported in other jurisdictions where PSA testing is common,18 but not in areas where testing is less prevalent.19
The sustained fall in mortality from prostate cancer that we observed in men aged 55–84 years is consistent with declines in 12 of 24 developed countries in which it has been studied.20 This fall began just 4 years after the start of widespread PSA testing; arguably 5–6 years earlier than would have been expected were screening solely responsible.17 A similar pattern has been observed for breast cancer mortality in NSW, with a 4-year lag between population-wide uptake of mammographic screening and an observable fall in mortality21 being somewhat earlier than randomised controlled trial outcomes would have predicted (though potentially confounded by improvements in treatment [tamoxifen]).
A pooled intention-to-screen analysis of two randomised controlled trials on PSA testing and prostate cancer mortality gave a relative risk of death in men randomly assigned to screening of 1.01 (95% CI, 0.80–1.29).2 High testing rates in the US and Australia have been followed closely by falls in mortality, but a similar fall has also been observed in the United Kingdom, where PSA testing is less common. Thus, neither experimental nor ecological studies have provided consistent evidence of reduced mortality in association with increased PSA testing.
There are other possible explanations for the improvement in mortality rate. Increased diagnosis of localised disease through increased PSA testing has led to more patients being offered curative treatment with surgery and radiotherapy. It is possible that improved techniques due to greater experience have resulted in better outcomes. A Swedish trial showed a lower risk of death from prostate cancer, all mortality, metastases and disease progression 10 years after diagnosis for men having surgery compared with watchful waiting.22 Patients operated on by surgeons with more experience appear to have lower risk of recurrence than patients of those with less experience.23
The use of luteinising hormone-releasing hormone agonists and non-steroidal anti-androgens rather than surgical castration for men with metastatic disease has been shown to result in small survival benefits.24 While hormonal treatment is unlikely to result in cure, longer survival will lead to greater competing causes of death and lower prostate cancer mortality rates.
Whatever its cause, the downward trend in prostate cancer mortality is clearly favourable; the upward trend in incidence may be less so. If it were solely due to earlier diagnosis of cancers that would otherwise be diagnosed later, the only harm would be lengthening the period in which men live with knowledge of their diagnosis. However, if it were also due to diagnosis of cancers that would never otherwise present clinically (over-diagnosis), there would be added harm from unnecessary diagnosis and treatment. Several studies have estimated over-diagnosis of prostate cancer caused by PSA testing,25 from 27%–56% in Europeans26 to 23%–34% in Americans.27 The range of these results includes the estimated 43% more men than expected who were diagnosed with prostate cancer between 1989 and 2005 in our study.
1 Observed (1972–2005) and projected (1989–2005) age-standardised prostate cancer incidence and mortality rates and age-standardised rates of PSA testing (1989–2006) in New South Wales*
 |
|
|
Received 17 December 2007, accepted 2 April 2008
- David P Smith1
- Rajah Supramaniam1
- Villis R Marshall2
- Bruce K Armstrong3
- 1 Cancer Council NSW, Sydney, NSW.
- 2 Surgical Specialties Service, Royal Adelaide Hospital, Adelaide, SA.
- 3 School of Public Health, University of Sydney, Sydney, NSW.
We thank the Public Health Division, NSW Health Department, for the provision of population data from the Health Outcomes Information and Statistical Toolkit (HOIST); the NSW Central Cancer Registry for provision of cancer data; and Warick Delprado of Douglass Hanly Moir Pathology for advice and data relating to pathology reporting.
None identified.
- 1. Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia 2000. Canberra: AIHW, 2003. (AIHW Cat. No. CAN 18.)
- 2. Ilic D, O’Connor D, Green S, Wilt T. Screening for prostate cancer: a Cochrane systematic review. Cancer Causes Control 2007; 18: 279-285.
- 3. Pinnock CB. PSA testing in general practice: can we do more now? Med J Aust 2004; 180: 379-381. <MJA full text>
- 4. Postma R, Schröder FH. Screening for prostate cancer. Eur J Cancer 2005; 41: 825-833.
- 5. Australian Health Technology Advisory Committee. Prostate cancer screening. Canberra: AGPS, 1996.
- 6. Urological Society of Australia and New Zealand. Screening for prostate cancer [fact sheet]. http://www.urosoc.org.au/consumer-information (accessed Apr 2007).
- 7. Medicare Australia. Medicare Benefits Schedule (MBS) item statistics reports. https://www.medicareaustralia.gov.au/statistics/mbs_item.shtml (accessed Nov 2007).
- 8. Australian Government Department of Health and Ageing. Medicare Benefits Schedule. http://www.health.gov.au/internet/mbsonline/publishing.nsf/Content/Medicare-Benefits-Schedule-MBS-1 (accessed Aug 2008).
- 9. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335-351.
- 10. Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int 2002; 90: 162-173.
- 11. Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia: an overview, 2006. Canberra: AIHW, 2007. (Cancer Series No. 37; AIHW Cat. No. CAN 32.)
- 12. Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA 1997; 277: 1452-1455.
- 13. Schröder FH, van der Cruijsen-Koeter I, de Koning HJ, et al. Prostate cancer detection at low prostate specific antigen. J Urol 2000; 163: 806-812.
- 14. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤ 4.0 ng per milliliter. N Engl J Med 2004; 350: 2239-2246.
- 15. Presti JC Jr, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol 2000; 163: 163-166.
- 16. Eichler K, Hempel S, Wilby J, et al. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol 2006; 175: 1605-1612.
- 17. Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer — part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst 1999; 91: 1017-1024.
- 18. Mullan RJ, Jacobsen SJ, Bergstralh EJ, et al. Decline in the overall incidence of regional-distant prostate cancer in Olmsted County, MN, 1980–2000. BJU Int 2005; 95: 951-955.
- 19. Mokete M, Shackley DC, Betts CD, et al. The increased rate of prostate specific antigen testing has not affected prostate cancer presentation in an inner city population in the UK. BJU Int 2006; 97: 266-269.
- 20. Baade PD, Coory MD, Aitken JF. International trends in prostate-cancer mortality: the decrease is continuing and spreading. Cancer Causes Control 2004; 15: 237-241.
- 21. Taylor R, Morrell S, Estoesta J, Brassil A. Mammography screening and breast cancer mortality in New South Wales, Australia. Cancer Causes Control 2004; 15: 543-550.
- 22. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005; 352: 1977-1984.
- 23. Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst 2007; 99: 1171-1177.
- 24. Damber JE. Decreasing mortality rates for prostate cancer: possible role of hormonal therapy? BJU Int 2004; 93: 695-701.
- 25. Bangma CH, Roemeling S, Schröder FH. Overdiagnosis and overtreatment of early detected prostate cancer. World J Urol 2007; 25: 3-9.
- 26. Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst 2003; 95: 868-878.
- 27. Telesca D, Etzioni R, Gulati R. Estimating lead time and overdiagnosis associated with PSA screening from prostate cancer incidence trends. Biometrics 2008; 64: 10-19.





Abstract
Objective: To describe trends in prostate-specific antigen (PSA) testing, prostate cancer incidence and mortality in New South Wales.
Design and setting: Descriptive analysis using routinely collected data of observed trends in PSA testing from 1989 to 2006, and prostate cancer cases and deaths from 1972 to 2005 in NSW.
Main outcome measures: Age-standardised and age-specific rates and joinpoint regression to identify changes in trends; projected trends observed before the introduction of PSA testing to quantify its impact on incidence and mortality rates.
Results: The number of PSA tests per year more than doubled between 1994 and 2006. Age-standardised incidence of prostate cancer peaked in 1994, fell by 10.0% per year to 1998 and then increased by 4.9% per year from 2001 to 2005. An estimated 19 602 (43%) more men than expected from preceding trends were diagnosed with prostate cancer between 1989 and 2005 after PSA testing was introduced. The incidence of recorded advanced prostate cancer at diagnosis fell from 13.0 per 100 000 men in 1987–1991 to 7.0 per 100 000 men in 2002–2005. The age-standardised mortality from prostate cancer increased by 3.6% per year between 1984 and 1990 and then fell by 2.0% per year to 2005.
Conclusions: There was a sustained increase in prostate cancer incidence in NSW after PSA testing was introduced. While falls in the incidence of advanced disease at diagnosis and mortality from prostate cancer after 1993 are consistent with a benefit from PSA testing, other explanations cannot be excluded.