Although lung cancer is not Australia’s most common cancer, it is the commonest cause of cancer death, with 7264 deaths recorded in 2004.1 The survival time of patients with lung cancer is among the poorest of any cancer, and it has only marginally improved in the past 15 years. In Victoria, 5-year relative survival increased slightly from 8% in 1990 to 11% in 2004.2 These grim statistics create a sense that treatment is ineffective, engendering a sense of nihilism among patients and their doctors, especially if the cancer cannot be surgically removed. As an example, a recently published book on interpreting cancer imaging includes the statement that “surgery offers the only chance of cure in non-small cell lung cancer”.3
Patients with locoregional non-small cell lung cancer (NSCLC) whose disease is inoperable are usually treated primarily with radiotherapy. For these patients, recent meta-analyses indicate that the combination of chemotherapy given concomitantly with radiotherapy is superior not only to radiotherapy alone4 but also to the combination of radiotherapy and chemotherapy administered sequentially.5 These studies demonstrated a statistically significant survival benefit at 3 years of 3.2% comparing concomitant chemoradiation with radiotherapy alone (16.6% v 13.4%), and 6.6% comparing concomitant chemoradiation with sequential radiotherapy and chemotherapy (24.8% v 18.2%).
While these survival benefits are important, longer-term benefits of treatment are also of interest to patients when making a decision about choice of treatment. In a survey of patients with a variety of advanced cancers, 85% wanted to know the longest possible survival time with treatment.6
Patient characteristics are shown in Box 1. There was no significant difference between the two studies in patient characteristics, except in ECOG performance status, with a greater proportion of asymptomatic patients (ECOG score = 0) enrolled in LURTCE than in LURTCF (62% v 20%, P = 0.027).
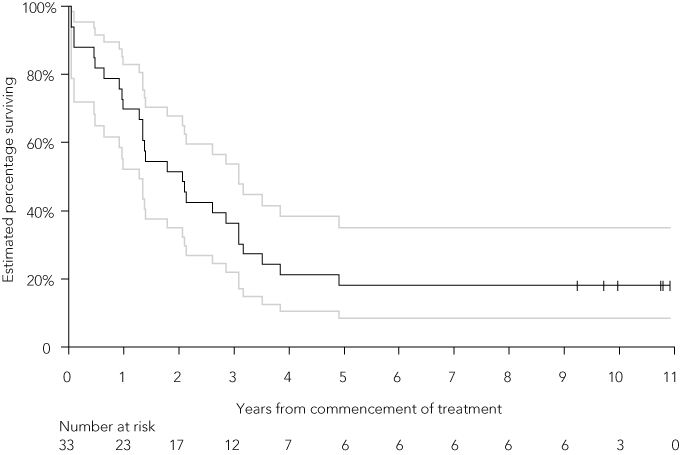
The overall survival of patients from both studies combined is shown in Box 2. The estimated median survival was 2.1 years (95% CI, 1.3–3.1 years). For the whole group, 2-year and 5-year survival rates were 52% (95% CI, 35%–68%) and 18% (95% CI, 8%–35%), respectively. There was no significant difference in overall survival between the two trials (P = 0.57). This conclusion remained the same after stratifying for ECOG performance status (P = 0.49).
A recently completed larger study (but with shorter follow-up) has reported similar survival rates. This was a trial in which 191 patients in one arm were randomly assigned to receive chemoradiation without surgery; estimated survival at 5 years for these patients was 20.3%.7
The term “cure” is a contentious one in oncology, and this is particularly true for NSCLC, where competing causes of death from other smoking-related comorbidities can confound the interpretation of survival data. Five-year survival probability is often used as a surrogate for “cure”, as relapse following radiotherapy-based treatment is unusual after this time, although it can occur.8 Informing patients of the longest observed survival time is an alternative way of presenting them with prognostic information. In a study of patient preferences in the setting of metastatic disease from a variety of cancers, patients more often wanted to know the longest survival time with treatment than the 5-year survival rate.6
It might be argued that in the absence of a control arm of untreated patients, the survival reported here may reflect the natural history of a carefully selected group of patients, rather than being a consequence of treatment. This seems unlikely, given that the 5-year relative survival of untreated patients with locally advanced NSCLC recorded in a large United States database ranged from 1% to 4%, depending on stage and histology.9 This is similar to the 5-year survival rate of 1% observed in patients treated with palliative radiotherapy.10
Received 9 April 2008, accepted 29 July 2008
- Nikki M Plumridge1
- Michael J Millward2
- Danny Rischin3
- Michael P MacManus1
- Andrew Wirth1
- Michael Michael3
- Kally Yuen4
- David L Ball1
- 1 Department of Radiation Oncology, Peter MacCallum Cancer Centre, Melbourne, VIC.
- 2 Sir Charles Gairdner Hospital, Perth, WA.
- 3 Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, VIC.
- 4 Biostatistics and Clinical Trials, Peter MacCallum Cancer Centre, Melbourne, VIC.
None identified.
- 1. Australian Institute of Health and Welfare. Australia’s health 2006. Canberra: AIHW, 2006. (AIHW Cat. No. AUS 73.) http://www.aihw.gov.au/publications/aus/ah06/ah06.pdf (accessed Mar 2008).
- 2. English D, Farrugia H, Thursfield VJ, et al. Cancer survival Victoria. Melbourne: The Cancer Council Victoria Epidemiology Centre, 2007. http://www.cancervic.org.au/downloads/about_our_research/canstats/cancer_survival_vic/Respiratory.pdf (accessed Mar 2008).
- 3. Hricak H, Husband JES, Panicek DM. Oncologic imaging: essentials of reporting common cancers. Philadelphia: Saunders, 2007: 76.
- 4. Rolland E, Le Chevalier T, Auperin A, et al. Sequential radio-chemotherapy (RT-CT) versus radiotherapy alone (RT) and concomitant RT-CT versus RT alone in locally advanced non-small cell lung cancer (NSCLC): two meta-analyses using individual patient data (IPD) from randomised clinical trials (RCTs) [abstract]. J Thorac Oncol 2007; 2 Suppl 4: S309-S310.
- 5. Auperin A, Rolland E, Curran WJ, et al. Concomitant radio-chemotherapy (RT-CT) versus sequential RT-CT in locally advanced non-small cell lung cancer (NSCLC): a meta-analysis using individual patient data (IPD) from randomised clinical trials (RCTs) [abstract]. J Thorac Oncol 2007; 2 Suppl 4: S310.
- 6. Hagerty RG, Butow PN, Ellis PA, et al. Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol 2004; 22: 1721-1730.
- 7. Albain KS, Swann RS, Rusch VR, et al. Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) vs CT/RT followed by surgical resection for stage IIIA(pN2) non-small cell lung cancer (NSCLC): outcomes update of North American Intergroup 0139 (RTOG 9309) [abstract]. J Clin Oncol 2005; 23 (16 Suppl): 624s.
- 8. MacManus MP, Wada M, Matthews JP, Ball DL. Characteristics of 49 patients who survived for 5 years following radical radiation therapy for non-small cell lung cancer: the potential for cure. Int J Radiat Oncol Biol Phys 2000; 46: 63-69.
- 9. Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: a national cancer data base report. Cancer 1999; 86: 1867-1876.
- 10. MacManus MP, Matthews JP, Wada M, et al. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer 2006; 106: 1110-1116.





Abstract
Objective: To measure long-term survival following combined chemotherapy and radiotherapy for inoperable non-small cell lung cancer.
Design and setting: Two prospective Phase I/II studies in the multidisciplinary Lung Service of a dedicated cancer hospital in Victoria, commencing in 1996 and 1997–1998.
Patients: 33 patients referred for treatment of histologically or cytologically proven inoperable non-small cell lung cancer, who had no evidence of distant metastases, Karnofsky performance status > 70%, weight loss < 10%, and no prior treatment for lung cancer. Patients were followed until death or for a minimum of 9 years.
Interventions: Patients in both studies were treated concomitantly with chemotherapy and radiotherapy 60 Gy in 30 fractions over 6 weeks. Chemotherapy in the first study (LURTCE) consisted of cisplatin and etoposide; in the second study (LURTCF), chemotherapy consisted of escalating doses of carboplatin and fluorouracil.
Main outcome measure: Overall survival.
Results: Six of 33 patients were still alive 9 years after commencement of treatment. Median survival for the whole group was 2.1 years (95% CI, 1.3–3.1 years), with 18% (95% CI, 8%–35%) of patients still alive at 5 years (plateau).
Conclusion: Long-term survival can be achieved in some patients with inoperable non-small cell lung cancer treated by radical chemoradiation alone, suggesting the possibility of cure.