Lactic acidosis is an acute abnormal metabolic state associated with substantial mortality.1 Although metformin therapy is listed among its causes, the evidence for this relationship is debated.2 The chemically related compound phenformin was withdrawn in the early 1970s because of a clear association with lactic acidosis,3 but metformin has different pharmacological properties, including a lack of effect on tissue lactate production in the fasting state.4 Epidemiological data do not support an association between metformin use and lactic acidosis: a recent large meta-analysis of prospective trials and observational cohort studies showed no cases of fatal or non-fatal lactic acidosis in 47 846 patient-years of metformin use.4
Such studies are likely to have respected contraindications and precautions, such as renal impairment, heart failure and a dosage of > 2 g per day, when metformin was prescribed. Case series, including one from Australia, have suggested that the use of the drug in the presence of one or more of these factors could increase the risk of lactic acidosis.5,6 However, survey data indicate that relatively large numbers of metformin-treated patients (one in four, or more) have at least one contraindication.7,8 Despite this, population-based rates of lactic acidosis do not appear to be associated with metformin use.9
The Fremantle Diabetes Study (FDS) took place in a postcode-defined population of 120 097 people in and around the port of Fremantle in Western Australia. Descriptions of recruitment, sample characteristics (including classification of diabetes type and details of patients not recruited) have been published.10 Of the 2258 patients with diabetes identified between April 1993 and 1996, 1426 (63%) were recruited to the FDS and 1294 had type 2 diabetes. Eligible patients who declined participation were a mean of 1.4 years older than participants, but their sex distribution, the proportion with type 2 diabetes and the distribution of treatment modalities were similar.10 The FDS protocol was approved by the Human Rights Committee at Fremantle Hospital, and all subjects gave informed consent before participation.
Recruited patients were invited to attend annual reviews until the FDS closed in 2001, 5 years after the last patient was recruited. The assessment at study entry and at each annual review included a comprehensive questionnaire and physical examination.10 In addition to details of all medical conditions and their management, demographic, socioeconomic and lifestyle data were recorded. Patients were requested to bring all medications to each visit, and details, including dosages, were recorded. Any missing medication data were collected by telephone or review of hospital case notes. Biochemical tests were performed on fasting blood and urine samples by using standard automated methods.
All hospital admissions in WA are recorded in the WA Data Linkage System (WADLS),11 which was used as a source of FDS patient outcomes from the beginning of the study in 1993 until 30 June 2006. All patients hospitalised with an International classification of diseases (ICD) code relating to acidosis were identified through this linkage, and the case notes of each patient were obtained and reviewed. Episodes of lactic acidosis were identified as those in which the patient had an arterial pH of < 7.35 in association with a venous or arterial plasma lactate > 5.0 mmol/L.1 Other data extracted for use in this analysis included the age at time of presentation, the dosage of metformin, the serum creatinine (from which we calculated the estimated glomerular filtration rate (eGFR) using the equation of the Modification of Diet in Renal Disease study12), and the presence or absence of comorbidities, including ischaemic heart disease, heart failure and respiratory failure.
We had complete data relating to oral hypoglycaemic drug use for 1279 FDS participants with type 2 diabetes (98.8 % of the sample) at the time of study entry. They were aged 64 years (95% CI, 39–84 years), 48.8% were men, and diabetes had been diagnosed a median 4.0 years (IQR, 1.0–9.0 years) previously. Of these patients, 425 (33.3%) were taking metformin (Box 1); of these, 18.4% had eGFR < 60 mL/min, 12.5% were taking > 2 g metformin daily, 5.2% had a history of hospitalisation for heart failure, and 2.8% had suffered a myocardial infarction within 6 months of assessment (Box 2). A total of 98 metformin-treated patients (23.1%) had at least one of these contraindications to its use.
Changes in contraindications and in metformin use over 5 years of follow-up (until study close in 2001) are shown in Box 1. There was a steady increase, from 33.3% to 55.1% over 5 years, in the percentage of patients prescribed metformin.
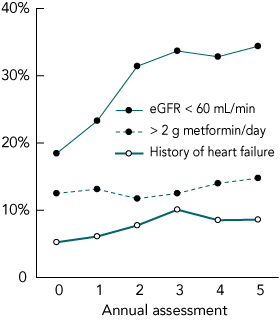
There were increases in the percentages of patients, of those prescribed metformin, with an eGFR < 60 mL/min (from 18.4% to 34.4%), who were taking > 2 g metformin daily (from 12.5% to 14.8%), or who had a history of hospitalisation for heart failure (from 5.2% to 8.6%) (Box 2). The percentage of those taking metformin and who had suffered a myocardial infarction within the previous 6 months fell to 0.3% over 5 years. Overall, the percentage of metformin-treated patients with at least one of these contraindications rose from 23.1% to 38.0% (Box 1).
Twenty-five cases of acidosis of any cause were identified from the WADLS data over the 13.2-year follow-up period between April 1993 and June 2006. Of these, five had all necessary diagnostic criteria for lactic acidosis (Box 3). Three of these five patients were taking metformin at the time of admission. All five patients had at least one significant comorbidity that can be associated with lactic acidosis (renal failure, myocardial infarction, cardiogenic shock or thromboembolic disease). Two patients died, one of whom was metformin-treated. Of the remaining 20 cases: 18 had definite biochemical evidence that the diagnosis was not lactic acidosis; and in two, there was inadequately documented biochemical characterisation of the nature of the acidosis.
None of the five confirmed cases of lactic acidosis occurred during the first of the follow-up periods, and so the incidence rate ratio for patients treated with metformin compared with those not treated with metformin was not calculable (Box 4).
During, long-term follow-up, the treatment-independent incidence was 5/12 466 patient-years or 40/100 000 patient-years (Box 4). Under the conservative assumption that the prevalence of metformin use was the same during the longer follow-up period as at the end of the FDS, the incidence of confirmed cases was 3/5228 patient-years in metformin-treated patients, or 57/100 000 patient-years. This is similar to 2/7238 patient-years (28/100 000 patient-years) in the non-metformin-treated group. The incidence rate ratio for confirmed cases of lactic acidosis among metformin-treated versus non-metformin-treated patients is, therefore, 0.48 (0.04 to 4.20) (P = 0.4) and the incidence rate difference is – 30 (– 105 to 46) (P = 0.4).
Metformin use increased during FDS follow-up, and it is likely to play an even greater role in the treatment of type 2 diabetes in future. Factors such as the characteristic therapeutic progression of type 2 diabetes and a reluctance to consider insulin at an early stage,13 together with evidence that metformin may be cardioprotective independently of its metabolic effects,14 help to explain these trends, but glycaemic targets are becoming more stringent,15 and increasing recent concerns over the adverse effects of thiazolidinediones16 could further promote metformin use. In addition, a recent European and United States consensus statement recommends that metformin should be commenced at the time of diagnosis, in concert with intensified lifestyle measures, because of its proven glycaemic efficacy in the absence of hypoglycaemia and weight gain.17
Increased metformin use in a population carries with it the risk that more patients will have contraindications, as seen in this study. Our data show that patients with renal impairment and heart failure had the greatest proportional increases in use. While this is likely to reflect, in part, a relationship between these complications and both increasing age and diabetes duration, it may also indicate that the relative benefits of metformin outweigh the risks when the prescriber is faced with a need for progressive therapeutic intensification. The relative temporal stability of the proportion of patients taking > 2 g/day is reassuring, given the evidence that dosages in this range are associated with little added glycaemic benefit but an increase in side effects.18
In surveys from Europe, the US and Canada, the estimated incidence of lactic acidosis in metformin-treated patients with type 2 diabetes has been 2–10/100 000 patient-years. The number of patient-years of observation in individual surveys has ranged from 22 296 to 2 893 900.19 The upper 95% confidence limits for the incidence of lactic acidosis in a Cochrane meta-analysis involving 47 846 patient-years in metformin-treated patients and 38 221 patient-years in non-metformin-treated patients were 6.3/100 000 and 7.8/100 000, respectively.4
Our data relating to episodes of lactic acidosis identified during follow-up before the close of the FDS in 2001 are consistent with these population and large-sample estimates. However, the mean age of our 793 surviving patients (62.0% of the cohort) at the close of the FDS, in 2001, was 72 (95% CI, 49–90) years, and their diabetes duration was 15.1 (IQR, 12.9–18.7) years. Increasing numbers were developing other potential causes of lactic acidosis, such as cardiovascular disease and renal impairment. This is reflected by the steadily rising proportion with metformin contraindications (Box 1). Consistent with this, lactic acidosis incidence rates were higher when the extended follow-up to 30 June 2006 was included. Nevertheless, values for incidence rate ratios and incidence rate differences did not suggest an increased lactic acidosis risk in metformin-treated patients compared with those taking other therapies for type 2 diabetes during up to 13.2 years of follow-up.
Our study had limitations. Compared with previous population-based surveys19 and systematic reviews,4 our total patient-years of follow-up were small, so there were relatively few events and wide associated confidence intervals. We estimate that about 150 000 patients would have been required to ensure that there was no type II error at 80% power. Nevertheless, strengths of the study include the detailed baseline characterisation of each patient, the fact that the WADLS captures both public and private hospital admissions in WA,11 that the rate of coding errors is low,20 and that there is a low rate of migration out of the state.21 In addition, we screened linked data for acidosis of any cause and reviewed the notes of each such case both to minimise the chance of missing an episode of lactic acidosis and to obtain the biochemical evidence necessary for diagnosis.
Given the apparent safety of metformin when prescribed according to the package insert,4 do the present data indicate that, as has been proposed,2 the validity of contraindications to metformin use should be reviewed? There is published evidence that metformin is safe in heart failure,22 renal insufficiency (serum creatinine up to 220 mmol/L),23 and in experimental models of sepsis.24 Some data even suggest benefit in the case of cardiac failure.25 On the basis of our results and other data,4 which do not show a higher rate of lactic acidosis in metformin-treated than non-metformin-treated type 2 diabetes, there is little evidence that metformin is an important cofactor for the development of lactic acidosis in patients with pre-existing major recognised precipitants, such as renal and heart failure. The recent Cochrane review stated that there is a need for large prospective, comparative trials in patients with type 2 diabetes and conditions that are currently considered contraindications to its use, such as chronic renal insufficiency.4 Assessment of the risk–benefit equation from such important trial data would require evaluation of the benefits of metformin therapy, such as reduced coronary heart disease14 as well as possible harms, such as lactic acidosis.
1 Percentages of patients in the Fremantle Diabetes Study taking metformin at each annual review, and percentages with and without contraindications to metformin use
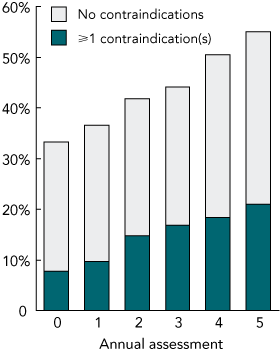
2 Percentages of patients in the Fremantle Diabetes Study, of those prescribed metformin, with renal failure, a history of heart failure, or taking a high dosage of metformin, at each annual review
 | |||||||||||||||
3 Patients from the Fremantle Diabetes Study with confirmed lactic acidosis
Myocardial infarction, acute renal failure, type 2 respiratory failure |
|||||||||||||||
Received 2 October 2007, accepted 2 January 2008
- Niklaus Kamber1
- Wendy A Davis2
- David G Bruce3
- Timothy M E Davis4
- School of Medicine and Pharmacology, University of Western Australia, Fremantle, WA.
We thank the FDS patients for their participation and the FDS staff for their help with collecting and recording clinical information. We thank the Biochemistry Department at Fremantle Hospital and Health Service for performing laboratory tests, and the diabetic education, podiatry and dietetic departments for assistance with recruitment of patients. The FDS was funded by the Raine Foundation, University of Western Australia. This substudy was funded by the Diabetes Research Foundation of Western Australia and an unrestricted educational grant from Alphapharm.
The funding sources had no role in the study design, data collection, analysis and interpretation, and writing or publication of this paper. Timothy Davis has received speaker fees from Alphapharm.
- 1. Stacpoole PW, Harman EM, Curry SH, et al. Treatment of lactic acidosis with dichloroacetate. N Engl J Med 1983; 309: 390-396.
- 2. Holstein A, Stumvoll M. Contraindications can damage your health — is metformin a case in point? Diabetologia 2005; 48: 2454-2459.
- 3. DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131: 281-303.
- 4. Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2006; (1): CD002967.
- 5. Misbin RI, Green L, Stadel BV, et al. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med 1998; 338: 265-266.
- 6. Nisbet JC, Sturtevant JM, Prins JB. Metformin and serious adverse effects. Med J Aust 2004; 180: 53-54. <MJA full text>
- 7. Emslie-Smith AM, Boyle DI, Evans JM, et al. Contraindications to metformin therapy in patients with type 2 diabetes — a population-based study of adherence to prescribing guidelines. Diabet Med 2001; 18: 483-488.
- 8. Calabrese AT, Coley KC, DaPos SV, et al. Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy. Arch Intern Med 2002; 162: 434-437.
- 9. Brown JB, Pedula K, Barzilay J, et al. Lactic acidosis rates in type 2 diabetes. Diabetes Care 1998; 21: 1659-1663.
- 10. Davis TM, Zimmet P, Davis WA, et al. Autoantibodies to glutamic acid decarboxylase in diabetic patients from a multi-ethnic Australian community: the Fremantle Diabetes Study. Diabet Med 2000; 17: 667-674.
- 11. Holman CD, Bass AJ, Rouse IL, Hobbs MS. Population-based linkage of health records in Western Australia: development of a health services research linked database. Aust N Z J Public Health 1999; 23: 453-459.
- 12. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461-470.
- 13. Davis TME, Davis WA, Bruce DG. Glycaemic levels triggering intensification of therapy in type 2 diabetes in the community: the Fremantle Diabetes Study. Med J Aust 2006; 184: 325-328. <MJA full text>
- 14. UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854-865.
- 15. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2006; 29 Suppl 1: S4-S42.
- 16. Solomon DH, Winkelmayer WC. Cardiovascular risk and the thiazolidinediones: déjà vu all over again? JAMA 2007; 298: 1216-1218.
- 17. Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm. Diabetologia 2006; 49: 1711-1721.
- 18. Garber AJ, Duncan TG, Goodman AM, et al. Efficacy of metformin in type II diabetes: results of a double-blind, placebo controlled, dose-response trial. Am J Med 1997; 103: 491-497.
- 19. Bailey CJ, Howlett HC. Defining the risk of lactic acidosis. In: Bailey CJ, Campbell IW, Chan JC et al, editors. Metformin: the gold standard. Chichester, UK: Wiley, 2007: 185-192.
- 20. Norman PE, Semmens JB, Laurvick CL, Lawrence-Brown M. Long-term relative survival in elderly patients after carotid endarterectomy: a population-based study. Stroke 2003; 34: e95-e98.
- 21. Bradshaw PJ, Jamrozik K, Jelfs P, Le M. Mobile Australians: a moving target for epidemiologists [letter]. Med J Aust 2000; 172: 566.
- 22. Roberts F, Ryan GJ. The safety of metformin in heart failure. Ann Pharmacother 2007; 41: 642-646.
- 23. Rachmani R, Slavachevski I, Levi Z, et al. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med 2002; 13: 428.
- 24. Gras V, Bouffandeau B, Montravers PH, Lalau JD. Effect of metformin on survival rate in experimental sepsis. Diabetes Metab 2006; 32: 147-150.
- 25. Eurich DT, Majumdar SR, McAlister FA, et al. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care 2005; 28: 2345-2351.





Abstract
Objective: To determine the incidence of lactic acidosis in community-based patients with type 2 diabetes, with special reference to metformin therapy.
Design: Substudy within a longitudinal observational study, the Fremantle Diabetes Study (FDS).
Participants and setting: 1279 patients from a postcode-defined population of 120 097 people in Western Australia.
Main outcome measures: Confirmed hospitalisation with lactic acidosis identified through the WA Data Linkage System during two periods: (1) from study entry, between 1993 and 1996, and study close in November 2001; and (2) from study entry to 30 June 2006.
Results: At entry, 33.3% of patients were metformin-treated, and 23.1% of these had one or more contraindications to metformin (55.1% and 38.0%, respectively, after 5 years’ follow-up). Five confirmed cases of lactic acidosis were identified during 12 466 patient-years of observation; all had at least one other potential cause, such as cardiogenic shock or renal failure. From study entry to close, the incidence was 0/100 000 patient-years in both metformin-treated and non-metformin-treated patients. Between study entry and 30 June 2006, incidence was 57/100 000 patient-years (95% CI, 12–168) in metformin-treated patients and 28/100 000 patient-years (95% CI, 3–100) in the non-metformin-treated group, an incidence rate difference of – 30 (– 105 to 46) (P = 0.4).
Conclusion: The incidence of lactic acidosis in patients with type 2 diabetes is low but increases with age and duration of diabetes, as cardiovascular and renal causes become more prevalent. Metformin does not increase the risk of lactic acidosis, even when other recognised precipitants are present.