Three patients with Australian parasitic myositis caused by the muspiceoid nematode Haycocknema perplexum are described. Treatment with albendazole led to a slow and incomplete recovery, but treatment with steroids caused life-threatening deterioration.
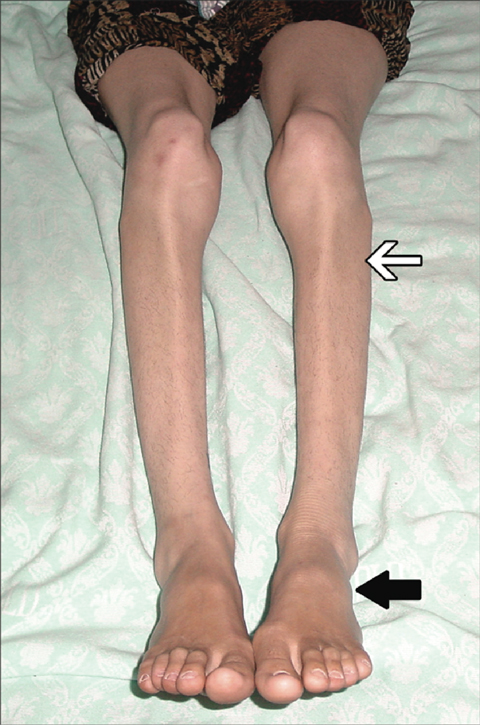
Although not acutely unwell or febrile, the patient was thin, with limb weakness and proximal and distal wasting. The facial muscles were wasted and weak, but palatal and ocular movements were normal. She was unable to stand from sitting without using her arms and had bilateral foot drop, but toe extension was preserved (Box 1). Reflexes were absent at the ankles and depressed elsewhere. Sensation appeared intact.
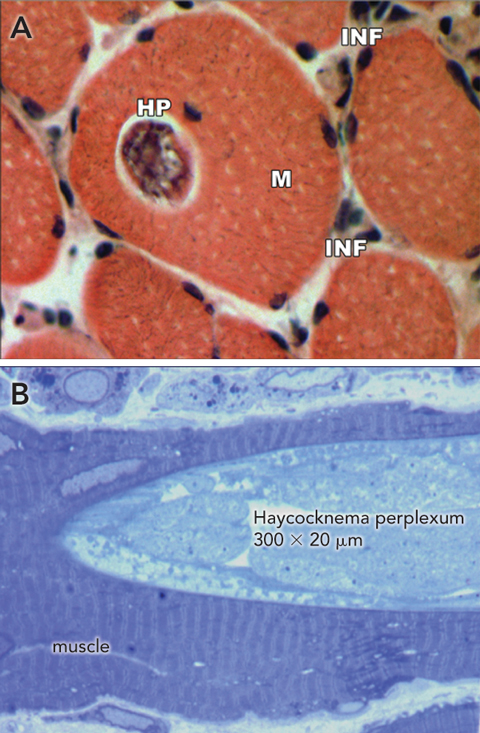
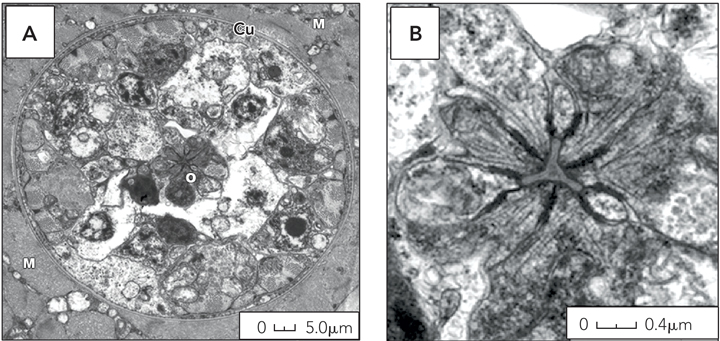
A biopsy of the quadriceps showed the muscle to be pale, flaccid and atrophic. On examination of paraffin sections, the muscle appeared highly abnormal; several muscle fibres contained sarcoplasmic nematode parasites characterised by a simple cephalic end and a sharply tapered caudal end. Unlike other nematodes that cause myositis, these parasites were not encysted. There was patchy interstitial and perivascular inflammation, predominantly with lymphocytes, and numerous necrotic fibres showing florid myophagia (Box 2). The parasite stained red with Gomori’s trichrome and positive with ATPase and cytochrome oxidase. Electron microscopy showed scattered sarcoplasmic parasites. Gravid female parasites were characterised by a thin cuticle, a central triradiated oesophageal lumen, and ova (Box 3). The parasite was identified as the nematode Haycocknema perplexum.1
Although H. perplexum myopathy has previously been reported, in two people from Tasmania,1,2 the patients we report had spent most or all of the past few years in tropical northern Australia. We contacted the physicans caring for the earlier patients, who confirmed that they, too, had been exposed to tropical Australia (Box 4). One had travelled extensively in Cape York, Far North Qld, and Kakadu National Park, Northern Territory, 5 years before diagnosis. Around that time, he developed a non-specific illness with raised liver transaminase levels, and liver biopsy showed mild reactive hepatitis with eosinophils. The other was a botanist who had visited the Northern Territory, Tasmania, Europe, Kenya and Indonesia on fieldwork before developing muscle weakness.
H. perplexum is a minute nematode measuring about 350 m by 20 m. The parasite appears to be able to complete its life cycle within human muscle, as adult nematodes are found within the sarcoplasm of muscle cells, while larvae are found both inside and outside myofibres.3 Electron microscopy shows the characteristic features of H. perplexum: a cuticularised triradiated oesophagus–intestine terminating in dark refringent granules (trophosomes).1 The outer cuticle is thin and has exterior corrugations. The male is shorter and narrower than the female, with the testes occupying 50%–60% of its body. The gravid female has paired uteri where 12 to 24 ova develop into larvae. Auto-reinfection is thought to occur when third-stage larvae escape by bursting through the female nematode’s body, a phenomenon known as endotokia matricida. The resulting damaged myofibres incite an intense inflammatory reaction resulting in myophagia.
H. perplexum was first described in the late 1990s, having been identified as the cause of a case of human myositis.2 The parasite is a member of the Robertdollfusidae family of Muspiceoidea nematodes. Distinct species of Muspiceoidea nematodes have been found in the tissues of various Australian vertebrates,4 including mice,3 bats,5 kangaroos and wallabies,6 and koalas.7 In animals, there is some evidence for cutaneous penetration as the mechanism of infection.3
- 1. Spratt DM, Beveridge I, Andrews JRH, Dennett X. Haycocknema perplexum n. g., n. sp. (Nematoda: Robertdollfusidae): an intramyofibre parasite in man. Syst Parasitol 1999; 43: 123-131.
- 2. Dennett X, Siejka SJ, Andrews JR, et al. Polymyositis caused by a new genus of nematode. Med J Aust 1998; 168: 226-227.
- 3. Spratt DM, Haycock P, Boyden JM, Nicholas WL. Aspects of the life history of Muspicea borreli (Nematoda: Muspiceidae), parasite of the house mouse (Mus domesticus) in Australia. Parasite 2002; 9: 199-205.
- 4. Spratt DM. Australian ecosystems, capricious food chains and parasitic consequences for people. Int J Parasitol 2005; 35: 717-724.
- 5. Bain O, Chabaud AG. Sur les Muspiceidae (Nématoda-Doryliamina) [French]. Ann Parasitol Hum Comp 1979; 54: 207-225.
- 6. Spratt DM, Speare R. Durikainema macropi gen. et sp. nov. (Muspiceoidea: Robertdollfusidae). A remarkable nematode from Macropodidae (Marsupialia). Ann Parasitol Hum Comp 1982; 57: 53-62.
- 7. Spratt DM, Gill PA. Durikainema phascolarcti n. sp. (Nematoda: Muspiceoidea: Robertdollfusidae) from the pulmonary arteries of the koala Phascolarctos cinereus with associated pathological changes. Syst Parasitol 1998; 39: 101-106.





We thank Dr Philip Marshall for clinical care of Patient 1 and for assistance with manuscript preparation.
None identified.