More than 90% of air passengers flying for more than 5 hours will develop some degree of ankle oedema.1 Use of low-compression hosiery (8–20 mmHg at the ankle) reduces subjectively rated flight-related symptoms of discomfort, swelling, fatigue, aching and tightness.2 However, the bulk of published research on flight-induced oedema results from studies that measure oedema with the incidence of symptomless deep vein thrombosis (DVT). Passengers can expect a reduction of both oedema and DVT when wearing below-knee compression stockings with ankle compression levels of 14–17 mmHg.3,4 The most recent Cochrane review on this subject suggests the need for further research on the relative effects of different pressures exerted by stockings.3 We initiated this trial because of the scarcity of reports on the effect of low-ankle-pressure graduated-compression tights (GCTs) on flight-induced ankle oedema and other flight-related symptoms.
The GCTs we used in our study comprised 76% nylon and Meryl microfibre, and 24% Roica spandex, and were developed by Australian sports physicians and sports scientists specifically to help athletes to recover more quickly from strenuous competition and training. They were full-leg-length gradient-compression stockings, similar (but with a lower compression) to antithrombosis stockings. The GCTs we used had the following pressures: about 5 mmHg at the ankle, 17–20 mmHg at the calf, and falling to 10 mmHg above the knee and 4 mmHg at the buttocks, compared with the frequently cited Scholl Flight Socks (SSL Australia Pty Ltd, Melbourne, Vic), which provide 14–17 mmHg of pressure at the ankle.4 GCTs promote blood flow from superficial veins into deep veins and compress deep veins.4-7
We conducted our unblinded, randomised crossover trial according to International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use/Guidelines for Good Clinical Practice (ICH GCP) standards,8 and the protocol was approved in advance by the Bellberry Human Research Ethics Committee.9 Each participant provided written informed consent before participating.
Participants were volunteers aged 18 years or older who had a confirmed flight booking on a flight of 5 or more hours’ continuous duration (with at least a 48-hour period between the forward and return flights) between 1 May and 8 October 2006. They included Qantas pilots referred by the Australian and International Pilots Association and general passengers who responded to advertisements in major metropolitan newspapers in Brisbane, or after being referred by other trial participants; volunteers were sought between 20 April 2006 and 16 September 2006. Volunteers were not eligible if they had received medical advice to wear GCTs in flight; had a previous history of DVT; had been prescribed medication for cardiovascular disease, coagulation disorders, varicose veins, bone or joint problems, diabetes or hypertension; had a body mass index (BMI) ≥ 35; or if they had had neoplastic disease within the previous 2 years (other than basal cell carcinomas). Thus, participants had a low to medium risk of DVT, and our study had the same inclusion and exclusion criteria as the LONFLIT 4 study.4
The GCT used was a Skins travel and recovery garment (Skins Compression Garments Pty Ltd, Sydney, NSW), listed on the Australian Register of Therapeutic Goods as a Class 1 medical device (ID: 80116). Participants were randomly assigned to wear low-ankle-pressure GCTs on either their forward or return flight. Random allocation was achieved by giving participants a sealed envelope with instructions according to a computer-generated randomisation sequence.10 During the control flight (when GCTs were not worn) participants wore their usual clothes.
Suggestions for in-flight exercises were given to both groups. The exercises were described on an instruction sheet,11 and included mild (mainly isometric) exercises and walking about the cabin and moving the legs for 3–4 minutes every hour.
The standard deviation of the difference was assumed to be 0.3 cm. The sample size required to detect a 0.2 cm difference in ankle size with 80% power and 5% significance was 38 participants.12 We estimated that 12 would drop out or be lost to follow-up, as the study was a self-assessment with no investigator visit. Thus, 50 participants were entered into the study.
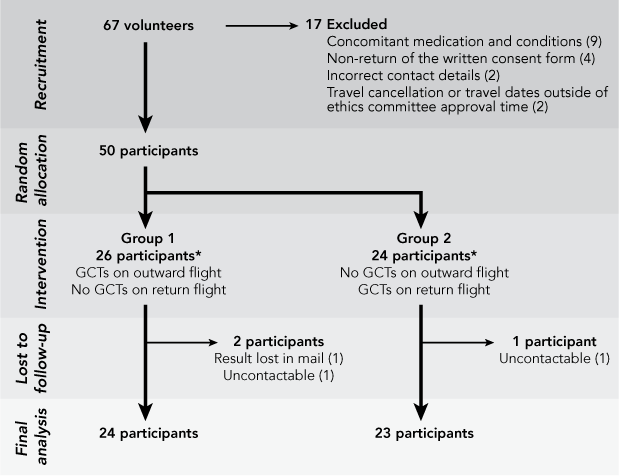
Sixty-seven people (23 Qantas pilots and 44 passengers) volunteered to participate in the study, and 17 (16 passengers and one pilot) were excluded (Box 1). Of the 50 participants (22 pilots and 28 passengers) recruited to the study, 26 wore GCTs on their outward flight (Group 1; 18 men and six women) and the remaining 24 wore GCTs on their return flight (Group 2; 17 men and six women). Ultimately, 47 participants (24 in Group 1 and 23 in Group 2) returned data and so completed the study (Box 1). Their ages ranged from 24 to 71 years.
Box 2 presents baseline characteristics for the participants in terms of physical characteristics, leg symptoms and activity level, and proportion of time at work spent sitting and standing. There is nothing in these data to suggest that our participants would not be representative of the general healthy flying population.
Measurements involving ratings were not analysed in a way that produced CIs of rating differences. Significant differences in Box 3 indicate a shift in the probability distributions of the ratings. So, for the rest of this section we make general observations on the ratings based on Box 3.
The use of GCTs to improve blood flow for patients with chronic venous insufficiency is routine. The rationale is based on the observation that oedema is the first symptom when venous blood flow is not adequately maintained and that compression stockings increase blood velocity by mechanically reducing the size of the larger veins. In addition to this, they also increase perivascular pressure and inhibit the outflow of blood and plasma factors from the endothelial venular gaps. This reduces contact with tissue factors and subsequent coagulation and thrombosis formation.13
Importantly, the 2006 Cochrane review established that compression garments worn during flight reduced oedema and the risk of symptomless DVT.3 Our findings show that low-ankle-pressure GCTs are effective at reducing flight-induced ankle oedema.
Our trial showed that even small quantitative increases in actual ankle circumference are associated with perceptions of leg swelling and associated pain and discomfort. This is consistent with the literature assessing compression stockings in healthy volunteers.14-16
Crew, and some passengers, actively work during flights. The impact from sleep disturbance (circadian rhythm disruption) and a reduced arterial partial pressure of oxygen (Pao2) have been implicated in reduced cognitive function in pilots17 and cabin crew.18 We explored self-assessed ratings of alertness, ability to concentrate and general energy levels in passengers. When wearing GCTs, participants had increased perceptions of alertness, ability to concentrate and higher energy levels compared with the flight where they did not wear GCTs. There are scarce data on passenger cognitive performance and recognition by research groups19 and other bodies of the need to conduct further studies.20
Visual analogue scales have been used to assess leg discomfort and swelling in flight attendants;2 however, only the pain intensity NRS adopted in this study has published reliability and validity in adults.21 While we made no objective tests of cognitive function, and unvalidated NRSs were used, our findings support improved cognitive function during flights when GCTs are worn, and further investigation is warranted.
Received 16 May 2007, accepted 11 September 2007
- Melissa J Hagan1
- Stephen M Lambert2
- 1 MPro, Brisbane, QLD.
- 2 The University Clinic, Westmead Hospital, Sydney, NSW.
Funding for this study was provided by Skins Compression Garments of Sydney, NSW. Melissa Hagan was contracted to conduct the study independently, and received no other funding from the company other than to conduct this research. Stephen Lambert is under contract as a scientific consultant for Skins Compression Garments. The study was initially designed by Stephen Lambert. Melissa Hagan independently collected, analysed and interpreted the data, and wrote the article. The agreement to publish the results was made prior to data collection with the Bellberry Human Research Ethics Committee, which approved this study. Skins Compression Garments had no influence over the decision to publish, and Melissa Hagan had final approval over the article’s content. The trial was registered with the Australian Clinical Trials Registry (trial no. 12606000150549) and was initiated on 19 April 2006.
- 1. Belcaro G, Geroulakos G, Nicolaides AN, et al. Venous thromboembolism from air travel: the LONFLIT study. Angiology 2001; 52: 369-374.
- 2. Weiss RA, Duffy D. Clinical benefits of lightweight compression: reduction of venous-related symptoms by ready-to-wear lightweight gradient compression hosiery. Dermatol Surg 1999; 25: 701-704.
- 3. Clarke M, Hopewell S, Juszczak E, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev 2006; (2): CD004002.
- 4. Belcaro G, Cesarone MR, Shah SS, et al. Prevention of edema, flight microangiopathy and venous thrombosis in long flights with elastic stockings. A randomized trial: the LONFLIT 4 Concorde Edema–SSL Study. Angiology 2002; 53: 635-645.
- 5. Byrne B. Deep vein thrombosis prophylaxis: the effectiveness and implications of using below-knee or thigh-length graduated compression stockings. Heart Lung 2001; 30: 277-284.
- 6. Jonker MJ, de Boer EM, Adèr HJ, Bezemer PD. The oedema-protective effect of Lycra support stockings. Dermatology 2001; 203: 294-298.
- 7. van Geest AJ, Franken CP, Neumann HA. Medical elastic compression stockings in the treatment of venous insufficiency. Curr Probl Dermatol 2003; 31: 98-107.
- 8. International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline. Guideline for good clinical practice. E6(R1). Current step 4 version (including the post step 4 corrections). 10 Jun 1996. http://www.ich.org/LOB/media/MEDIA482.pdf (accessed Dec 2007).
- 9. Bellberry Limited. Supporting research and ethics. http://www.bellberry.com.au/ (accessed Dec 2007).
- 10. McGraw Hill. Vadum Rankin. Statistical applets. Random assignment. http://www.assumption.edu/users/avadum/applets/applets.html (accessed Mar 2006).
- 11. QANTAS. Your health inflight. http://www.qantas.com.au/info/flying/inTheAir/yourHealthInflight (accessed Mar 2006).
- 12. Schoenfeld DA. Find statistical considerations for a cross-over study where the outcome is a measurement. http://hedwig.mgh.harvard.edu/sample_size/quan_measur/cross_quant.html (accessed Mar 2006).
- 13. Schreijer AJM, Cannegieter SC, Meijers JCM, et al. Activation of coagulation system during air travel: a crossover study. Lancet 2006; 367: 832-838.
- 14. Piller NA, Moseley A, Fauser F, et al. A randomised, single-blinded, cross-over trial of the effectiveness of below the knee support socks (stockings) for workers who stand for prolonged periods of time. Final report. Adelaide: WorkCover South Australia, 2004.
- 15. Kraemer WJ, Volek JS, Bush JA, et al. Influence of compression hosiery on physiological responses to standing fatigue in women. Med Sci Sports Exerc 2000; 32: 1849-1858.
- 16. De Boer EM, Broekhuijsen RW, Nieboer C, et al. Lycra support tights: are they effective? Phlebology 1999; 14: 162-166.
- 17. Johnson NR, Rantanen EM. Objective pilot performance measurement: a literature review and taxonomy of metrics. Proceedings of the 13th International Symposium of Aviation Psychology; 2005 Apr 18-21, Oklahoma City, USA. http://www.humanfactors.uiuc.edu/Reports &PapersPDFs/isap05/johranavpsy05.pdf (accessed Dec 2007).
- 18. Gander PH, Gregory KB, Miller DL, et al. Flight crew fatigue V. Long haul air transport operations. Aviat Space Environ Med 1998; 69 (9, Section II, Suppl): B37-B48.
- 19. ACER CoE. Airliner Cabin Environment Research [website]. http://acer.eng.auburn.edu/research. html (accessed Dec 2007).
- 20. National Research Council, Committee on Air Quality in Passenger Cabins of Commercial Aircraft. The airliner cabin environment and the health of passengers and crew. Washington, DC: National Academy Press, 2002.
- 21. Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003; 106: 337-345.





Abstract
Objective: To determine if low-ankle-pressure graduated-compression tights (GCTs) reduce flight-induced ankle oedema and subjectively rated travel symptoms of leg pain, discomfort and swelling, and improve energy levels, ability to concentrate, alertness, and post-flight sleep.
Design, setting and participants: Open, randomised crossover trial comparing the effects of GCTs (5 mmHg at ankle, 17–20 mmHg at calf and falling to 10 mmHg above knee and 4 mmHg at buttocks) among 50 adults on flights of 5 hours’ or more duration between 1 May and 8 October 2006; 47 volunteers (pilots and passengers) completed the trial.
Main outcome measures: Differences in right ankle circumference before and after flight with GCTs and without GCTs; travel symptoms rated on visual analogue scales.
Results: Low-ankle-pressure GCTs decreased ankle swelling (mean difference, − 0.19 cm; 95% CI, − 0.33 to − 0.65 cm; P = 0.012). Participants reported their legs felt better (mean, 1.6; P < 0.001; 95% CI, 1.0 to 2.1), warmer (mean, − 1.1; P < 0.001; 95% CI, − 1.6 to − 0.6), and they had a better night’s sleep (mean, 1.2; P < 0.001; 95% CI, 0.8 to 1.7) after the flight when they wore GCTs. Shifts in rating-scale probability distributions showed improvements in the ratings of pain (60%; P < 0.001), leg discomfort (50%; P = 0.001), leg swelling (45%; P = 0.006), energy levels (18%; P = 0.016), alertness levels (13%; P = 0.031), and concentration (12%; P = 0.023) when wearing GCTs.
Conclusions: Low-ankle-pressure GCTs reduce flight-induced ankle oedema and subjectively rated travel symptoms of leg pain, discomfort and swelling, and improve energy levels, ability to concentrate, alertness, and post-flight sleep.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12606000150549.