Clinical trial evidence supports routine invasive management of acute coronary syndromes (ACS), with coronary angiography and subsequent revascularisation when deemed clinically appropriate. Data from these trials show reduced mortality with primary percutaneous coronary intervention (PCI) for ST-segment-elevation myocardial infarction (STEMI), and reductions in composite ischaemic events among patients receiving successful medical reperfusion, failed medical reperfusion, and those presenting with high-risk ACS without initial ST-segment elevation.1-3
However, the provision of routine early angiography and revascularisation in ACS management remains challenging. Such an approach requires access to cardiac catheterisation laboratories, the availability of trained staff, and adequately designed clinical networks to cope with the burden of ACS in the community. In Australia, this is made more complex by the issue of geographical distances requiring greater resource commitments. Being able to demonstrate the clinical effectiveness of the early invasive strategy for ACS management in local settings would help gain broader support for this resource-intensive strategy.
Therefore, within a prospective national registry of patients with ACS, we sought to document the 12-month case fatality rates for various ACS presentations, and explore the relationship between an invasive strategy and late mortality.
We conducted the Acute Coronary Syndrome Prospective Audit (ACACIA, protocol number PM_L_0051) between 1 November 2005 and 31 July 2007, involving 39 hospitals across all states and territories of Australia. These sites were selected to be representative of rural (25%) and metropolitan (75%) centres, interventional (83%) and non-interventional (17%) centres, and 52% of sites reported onsite cardiac surgical services. Each site sought consecutive enrolment of between 100 and 150 patients admitted from the local emergency service for suspected ACS (median, 99). Patients presenting with ACS thought to be secondary to major trauma or surgery were excluded.
Patients transferred into study centres were excluded if more than 12 hours had passed since their initial presentation, to enable more accurate assessment of immediate care.
Ethics committee approval was provided at each site. Informed consent was obtained from all patients except for those who died before consent was sought — access to their medical records was granted by the local ethics committees.
Patients presenting with suspected STEMI, high-risk and intermediate-risk non-ST-segment-elevation ACS (NSTEACS) as defined by the National Health Data Dictionary (national standardised definitions set held by the Australian Institute of Health and Welfare) risk classification were eligible for enrolment, details of which have been described elsewhere.4-6 Allocation to each risk stratum was centrally adjudicated to ensure consistency of enrolment criteria. The primary discharge diagnosis was determined by investigators at each site, but confirmed by a central query process. Allocation to “non-cardiac chest pain” was made when ACS was excluded but no specific alternative diagnosis was made, while allocation to “other” diagnoses was made when an alternative diagnosis was provided. Analyses in this study reflect the discharge diagnosis.
Data pertaining to demographic, clinical, procedural, temporal and logistical para-meters involved in the management of ACS patients were obtained. These variables focused on hospital characteristics, clinical risk factors, the time to various aspects of medical care, and the distance travelled for patients transferred for invasive procedures. The use of various medications, including antithrombotic agents, statins, angiotensin-converting enzyme (ACE) inhibitors/angiotensin-receptor (AR) antagonists, and β-blockers, in hospital, at discharge, at 6 months and 12 months were also assessed.
Early invasive management was defined as angiography at any time within the acute hospital stay, regardless of transfer between acute care hospitals. Patients discharged home or to chronic care facilities who subsequently underwent outpatient angiography were not considered to have had early invasive management. The use and timing of PCI, and coronary artery bypass grafting (CABG) were also recorded. All data were collected by trained clinical trial coordinators.
Standard definitions consistent with the National Health Data Dictionary were used for inhospital events.5 Specifically, myocardial infarction (MI) required a rise in biomarker levels greater than the local threshold definition for troponin and/or more than twice the upper limit of normal for creatine kinase-MB (CK-MB) isoenzyme (in the absence of a CK-MB level, creatine kinase level was used). Recurrent MI required a further > 25% rise in troponin level or > 50% rise in CK-MB level, more than 24 hours after admission. Following PCI and CABG, a level of CK-MB > 3 times (for PCI) and > 5 times (for CABG) the upper limit of normal within 48 hours of the procedure or new Q waves was required. Stroke was determined by investigators, with cerebral imaging reports sought where possible.
All-cause mortality was determined during the index hospitalisation, at 6 months, and at 12 months. Among patients reported as lost to follow-up by the investigating site, a query to the Australian Institute of Health and Welfare National Death Register was undertaken to confirm vital status and cause of death. Data on late non-fatal recurrent acute coronary events, stroke and coronary revascularisation was obtained from hospital discharge summaries and diagnosis-related group (DRG) coding reports.
Demographic, clinical, procedural factors and late outcomes are presented, stratified by discharge diagnosis, focusing on patients with a “coronary” diagnosis (STEMI, non-STEMI, unstable angina and stable angina). Normally distributed variables are expressed as mean (± SD) and non-Gaussian factors are reported as median (and interquartile range [IQR]). Counts are presented as number and percentage. We used χ2 tests for comparisons of binary outcomes between groups. Kaplan–Meier survival curves, stratified by discharge diagnosis, were plotted and compared by log rank test. Assessment of late compliance was confined to patients without stated contraindications who survived to 12 months.
To evaluate the impact of invasive management on 12-month mortality, a propensity analysis was conducted.7 A non-parsimonious logistic regression model describing the propensity for inpatient invasive management was developed, including patient characteristics, past history and co-morbid conditions as well as the characteristics of the physician and hospital for each patient’s initial presentation. Interactions between these variables were also explored. Twenty-nine patients undergoing PCI or CABG without prior angiography because of known anatomy were excluded. This model demonstrated a high predictive capacity with a c-index of 0.853 (Hosmer–Lemeshow goodness-of-fit test, P = 0.487).
Among patients with ACS who survive to hospital discharge, the association between inpatient angiography and 12-month mortality was then assessed through Cox proportional hazards modelling, adjusting for key clinical covariates (GRACE [Global Registry of Acute Coronary Events] risk score, age, Killip class, renal function, diabetes, prior MI, prior cardiac failure, prior CABG, statin therapy, ACE-inhibitor therapy, stratified by admission diagnosis), and the propensity score as a continuous variable, with and without the inclusion of inpatient revascularisation. The proportional hazards assumption was assessed for each covariate. The effect of GRACE score varied with time and was therefore was entered into the model as a time-varying covariate. A probability of < 0.05 was considered statistically significant. All analyses were performed with Stata, version 9.1 (StataCorp, College Station, Tex, USA).
Among the 3402 patients enrolled, 12-month vital status was confirmed for 3393 patients (99.7%). Of the nine patients lost to follow-up, consent was withdrawn for five patients and no follow-up was available for four patients. Seven hundred and fifty-five (22.3%) were admitted with suspected STEMI, while 1942 (57.2%) were considered to have high-risk and 696 (20.5%) were considered to have intermediate-risk NSTEACS. Almost a quarter of patients (810, 23.9%) were enrolled from non-metropolitan centres and 119 (3.5%) were Indigenous. The median age was 65.5 years (IQR, 55.3–75.1 years), while 1202 (35.4%) were women and 886 (26.1%) had diabetes. An estimated creatinine clearance of < 60 mL/min/1.73 m2 was observed in 923 (27.2%) of the patients. A history of coronary artery disease was reported in 1673 (49.3%), while prior CABG and PCI were recorded in 492 (14.5%) and 606 (17.9%) patients, respectively. By discharge, after excluding patients lost to follow-up, 716 (21.1%), 1025 (30.2%), 812 (23.9%) and 137 (4.0%) patients were diagnosed with STEMI, non-STEMI, unstable angina and stable angina, respectively, while 528 (15.6%) and 175 (5.2%) patients were discharged under the diagnosis of non-cardiac chest pain and “other” diagnoses (Box 1). The transition from the initial working diagnosis to final diagnosis is shown in Box 2.
Invasive management, including subsequent coronary revascularisation during the index hospitalisation, was more common among patients discharged with STEMI compared with other patients. The use of clinical guideline-recommended medications was also more frequent among these patients (Box 3). Revascularisation after the index admission was observed in 322 patients (9.5%), at a median time of 63 days (IQR, 26–137 days). Over 12 months, a loss of compliance was evident with all of the medications, except for ACE inhibitors or AR antagonists. This decline was most prominent with clopidogrel (Box 4).
Factors most strongly associated with invasive management during the index hospitalisation were the hospital having an onsite cardiac surgical service, patients being admitted with high-risk NSTEACS and patients presenting with suspected STEMI. Clinical factors associated with conservative management included diabetes, reduced renal function, prior MI, prior CABG, prior heart failure, and a known history of congestive cardiac failure. For each decade above the median age, patients were 38.7% less likely to receive invasive management during the index hospitalisation. Patients enrolled at a non-metropolitan centres were also less likely to receive invasive management (Box 5).
Twelve-month survival by discharge diagnosis is presented in Box 6. Mortality rates among patients with MI were similar, regardless of ST-segment changes at the time of presentation. (STEMI, 57/716 [8.0%] v NSTEMI, 108/1025 [10.5%] v unstable angina, 27/812 [3.3%] v stable angina, 5/137 [3.7%]; P < 0.001 and STEMI v NSTEMI, P = 0.071) Recurrent MI, and late coronary revascularisation were more common in the high-risk cohort. Twelve patients discharged with non-cardiac chest pain (2.3%) and nine discharged with other diagnoses (5.2%) had died by 12 months. Box 7 shows outcomes to 12 months for patients discharged with a coronary diagnosis.
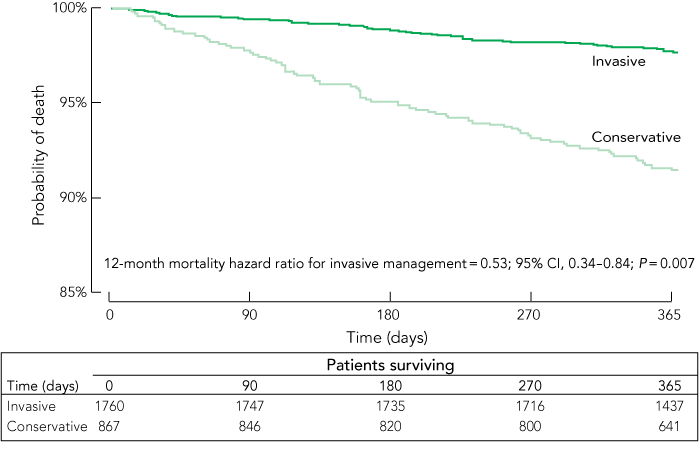
Patients receiving invasive management during the index hospitalisation experienced a lower rate of late mortality compared with patients treated conservatively (invasive, 3.7% v conservative, 10.1%; P < 0.001). This relationship persisted even when the analysis was restricted to patients discharged alive with a coronary diagnosis — STEMI, NSTEMI, unstable angina and stable angina (hazard ratio [HR], 0.25; 95% CI, 0.17–0.36; P < 0.001). However, invasive management was also correlated with lower risk and more prescription of guideline medications (Box 8).
Box 9 shows survival curves for invasive and conservative management after adjustment for the propensity score and other important confounders; invasive management was associated with an HR for 12-month mortality of 0.53. This benefit was driven by revascularisation. When the performance of either PCI or CABG during the index hospitalisation was adjusted for, angiography alone was no longer significantly associated with survival (HR, 0.84; 95% CI, 0.53–1.32; P = 0.477) while the HR for revascularisation was 0.30 (95% CI, 0.16–0.56; P < 0.001).
This study represents the largest ACS registry conducted exclusively within Australia to date. It provides not only a unique perspective on the clinical characteristics, management and late clinical outcomes of Australian patients, but also the opportunity to explore the clinical and geographical factors associated with the provision of care, particularly invasive management. We observed: (i) a late mortality rate among patients presenting with MI of around 9%, regardless of ST-segment status at the time of presentation; (ii) a persistent burden of recurrent MI and late revascularisation; (iii) incomplete provision of evidence-based therapies; and (iv) a relative mortality advantage associated with providing invasive management among ACS patients.
Within the era of evidence-based medicine, early mortality rates among ACS patients have declined.8 However, inhospital mortality rates are a poor reflection of later mortality among these patients. Within our broad cohort drawn from all states and territories, one in 11 patients with the diagnosis of MI had died by 12 months. We observed little difference in late mortality rate among patients presenting with or without ST-segment elevation, as seen with other international registries.9 In addition, these patients continued to experience a substantial burden of non-fatal recurrent ischaemic events, in particular, a high rate of late revascularisation. Whether these clinical events represent recurrent ischaemia in the context of an initial conservative strategy or planned delayed invasive management is uncertain.
Despite the substantial clinical trial evidence supporting early invasive management for high-risk ACS patients, application of this evidence in Australia appears incomplete. In contrast with patients discharged with the diagnosis of STEMI, 90% of whom had an assessment of their coronary vasculature (angiography and/or PCI), only 71% of patients discharged with NSTEMI and 45% of those discharged with unstable angina underwent invasive management before discharge. As seen in other studies, factors such as age, sex, and renal function influence this clinical decision.10,11 Furthermore, as would be expected given the national distribution of health services, we observed that onsite clinical services, and rural versus metropolitan hospital location influenced the provision of invasive management.
Consistent with trial evidence, but of greater magnitude, was the relationship between undergoing early invasive management and mortality, even after adjustment for other factors known to influence late outcome. These data reinforce the importance of delivering invasive management to all patients presenting with high-risk ACS. Furthermore, objective assessment of the proportion of patients undergoing invasive management represents a valuable measure for assessing quality of care and the effectiveness of regional health care systems.
However, the discordance between evidence from clinical trials and registries with regard to reduced mortality with invasive management requires careful consideration.12 The “correlation” between the provision of other guideline-recommended therapies and invasive management among lower-risk patients is an important observation.13 We found that, on average, patients in our study who underwent invasive management received a better total package of care. While the “propensity” model for angiography demonstrated high discriminatory capacity (c-index, 0.853; Box 5), a benefit persisted even after adjusting for this factor and other known predictors of late mortality, such as receipt of other guideline therapies. Our analyses should not be interpreted as diminishing the importance of such therapies. Clearly, the most obvious possible explanation is that there are unmeasured but clinically appreciated factors that influence the decision not to undertake early angiography, and these factors are very powerful in their effect on late mortality. Furthermore, these unmeasured factors must be very prevalent, and more common among patients presenting with NSTEMI and unstable angina than those with STEMI. An alternative explanation is also plausible and likely to be working in concert with the incomplete adjustment mentioned above. Analyses of registry data have documented the “lower-risk” and “better-treated” nature of patients randomised in clinical trials.14,15 In this context, any therapy is likely to demonstrate a more modest relative benefit. When extending treatment strategies to higher-risk populations beyond those studied in clinical trials, a greater impact may be expected, hence widening the observed treatment effect. Therefore, while adjustment for physician selection is likely to be incomplete, even after propensity adjustment, a proportion of the late mortality observed in this registry is likely to be preventable by more complete application of the early invasive approach to ACS management in Australia.
1 Patient characteristics by discharge diagnosis
Characteristic |
STEMI (n = 716) |
NSTEMI (n = 1025) |
Unstable angina (n = 812) |
Stable angina (n = 137) |
Non-cardiac chest pain (n = 528) |
Other (n = 175) |
|||||||||
Mean age in years (SD) |
62.1 (19.9) |
68.4 (20.2) |
68.1 (18.1) |
65.1 (18.7) |
60.0 (22.0) |
69.1 (18.8) |
|||||||||
Female |
181 (25.3%) |
333 (32.5%) |
289 (35.6%) |
65 (47.4%) |
251 (47.5%) |
83 (47.4%) |
|||||||||
Diabetes |
134 (18.7%) |
289 (28.2%) |
260 (32.0%) |
40 (29.2%) |
110 (20.8%) |
53 (30.3%) |
|||||||||
Hypertension |
358 (50.0%) |
673 (65.7%) |
600 (73.9%) |
103 (75.2%) |
313 (59.3%) |
118 (67.4%) |
|||||||||
Dyslipidaemia |
326 (45.5%) |
595 (58.0%) |
627 (77.2%) |
90 (65.7%) |
299 (56.6%) |
95 (54.3%) |
|||||||||
Current smoking |
237 (33.1%) |
234 (22.8%) |
130 (16.0%) |
31 (22.6%) |
109 (20.6%) |
32 (18.3%) |
|||||||||
Family history of CAD |
233 (32.5%) |
331 (32.3%) |
237 (29.2%) |
39 (28.5%) |
151 (28.6%) |
43 (24.6%) |
|||||||||
Prior myocardial infarction |
97 (13.5%) |
288 (28.1%) |
343 (42.2%) |
43 (31.4%) |
102 (19.3%) |
51 (29.1%) |
|||||||||
Prior PCI |
75 (10.5%) |
144 (14.0%) |
259 (31.9%) |
31 (22.6%) |
74 (14.0%) |
23 (13.1%) |
|||||||||
Prior CABG |
22 (3.1%) |
160 (15.6%) |
202 (24.9%) |
22 (16.1%) |
55 (10.4%) |
31 (17.7%) |
|||||||||
Prior stroke |
23 (3.2%) |
75 (7.3%) |
70 (8.6%) |
16 (11.7%) |
24 (4.5%) |
19 (10.9%) |
|||||||||
Known PAD |
24 (3.4%) |
83 (8.1%) |
55 (6.8%) |
6 (4.4%) |
16 (3.0%) |
10 (5.7%) |
|||||||||
Prior atrial fibrillation |
35 (4.9%) |
125 (12.2%) |
144 (17.7%) |
12 (8.8%) |
55 (10.4%) |
46 (26.3%) |
|||||||||
Median creatinine clearance rate in mL/min (25th–75th percentile) |
74.5 |
70.8 |
74.7 |
72.9 |
78.8 |
68.4 |
|||||||||
Mean white cell count (SD) |
10.8 (4.6) |
8.7 (3.8) |
7.7 (3.0) |
7.7 (2.8) |
7.6 (3.0) |
9.4 (3.9) |
|||||||||
Median GRACE score (25th–75th percentile) |
144 |
135 |
112 |
107 |
95.5 |
131 |
|||||||||
STEMI = ST-segment-elevation myocardial infarction. NSTEMI = non-ST-segment-elevation myocardial infarction. CAD = coronary artery disease. PCI = percutaneous coronary intervention. CABG = coronary artery bypass grafting. PAD = peripheral artery disease. GRACE = Global Registry of Acute Coronary Events (higher score implies greater risk). |
|||||||||||||||
2 Transition of patients from admission (working) diagnosis to final diagnosis
|
Admission diagnosis |
||||||||||||||
|
Suspected STEMI |
High-risk NSTEACS |
Intermediate-risk NSTEACS |
||||||||||||
Total patients |
756 |
1948 |
698 |
||||||||||||
Final diagnosis |
|
|
|
||||||||||||
STEMI |
708 (93.6%) |
8 (0.4%) |
1 (0.1%) |
||||||||||||
NSTEMI |
17 (2.2%) |
992 (50.9%) |
18 (2.6%) |
||||||||||||
Unstable angina |
10 (1.3%) |
549 (28.2%) |
256 (36.7%) |
||||||||||||
Stable angina |
3 (0.4%) |
73 (3.7%) |
61 (8.7%) |
||||||||||||
Non-cardiac pain |
7 (0.9%) |
210 (10.8%) |
313 (44.8%) |
||||||||||||
Other |
11 (1.5%) |
116 (6.0%) |
49 (7.0%) |
||||||||||||
Lost to follow-up |
1 |
6 |
2 |
||||||||||||
STEMI = ST-segment-elevation myocardial infarction. NSTEACS = non-ST-segment-elevation acute coronary syndrome. NSTEMI = non-ST-segment-elevation myocardial infarction. |
|||||||||||||||
3 Administration of clinical guideline-recommended medications, and angiography and revascularisation among patients discharged with a coronary diagnosis
Treatment |
STEMI (n = 716) |
NSTEMI (n = 1025) |
Unstable angina (n = 812) |
Stable angina (n = 137) |
P |
||||||||||
Aspirin |
648 (90.5%) |
906 (88.4%) |
683 (84.1%) |
111 (81.0%) |
< 0.001 |
||||||||||
Clopidogrel |
571 (79.7%) |
644 (62.8%) |
408 (50.2%) |
62 (45.3%) |
< 0.001 |
||||||||||
β-Blockers |
563 (78.6%) |
741 (72.3%) |
549 (67.6%) |
86 (62.8%) |
< 0.001 |
||||||||||
ACE-inhibitor or AR-antagonist |
571 (79.7%) |
722 (70.4%) |
523 (64.4%) |
93 (67.9%) |
< 0.001 |
||||||||||
Statin |
639 (89.2%) |
876 (85.5%) |
676 (83.3%) |
103 (75.2%) |
< 0.001 |
||||||||||
Angiography |
642 (89.7%) |
726 (70.8%) |
365 (44.8%) |
49 (35.8%) |
< 0.001 |
||||||||||
PCI |
509 (71.1%) |
349 (34.0%) |
116 (14.3%) |
12 (8.8%) |
< 0.001 |
||||||||||
Stent* |
484 (95.1%†) |
340 (97.4%†) |
114 (98.3%†) |
12 (100.0%†) |
0.163 |
||||||||||
CABG |
45 (6.3%) |
101 (9.9%) |
54 (6.7%) |
2 (1.5%) |
< 0.001 |
||||||||||
STEMI = ST-segment-elevation myocardial infarction. NSTEMI = non-ST-segment-elevation myocardial infarction. ACE = angiotensin-converting enzyme. AR = angiotensin receptor. PCI = percutaneous coronary intervention. CABG = coronary artery bypass grafting. * At least one stent among patients undergoing PCI during their index hospitalisation. † Percentages are of patients undergoing PCI during their index hospitalisation. |
|||||||||||||||
4 Compliance with guideline-recommended medications among patients who survived to 12 months
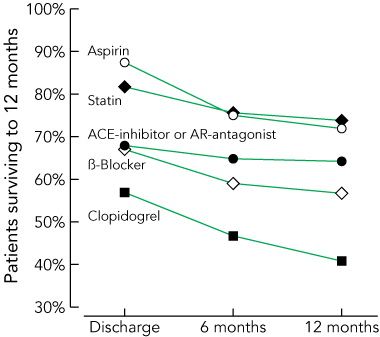
ACE = angiotensin-converting enzyme. AR = angiotensin receptor. |
5 Factors associated with invasive management during patients’ index hospital admission
Factor |
Odds ratio (95% CI) |
P |
|||||||||||||
Estimated glomerular filtration rate < 30 mL/min/173 m2 |
0.35 (0.21–0.60) |
< 0.001 |
|||||||||||||
Prior congestive cardiac failure |
0.39 (0.28–0.56) |
< 0.001 |
|||||||||||||
Non-metropolitan hospital |
0.47 (0.35–0.62) |
0.044 |
|||||||||||||
Prior coronary artery bypass grafting |
0.48 (0.36–0.62) |
< 0.001 |
|||||||||||||
History of diabetes |
0.60 (0.49–0.75) |
< 0.001 |
|||||||||||||
History of chronic obstructive pulmonary disease |
0.69 (0.50–0.94) |
0.022 |
|||||||||||||
History of coronary artery disease |
0.71 (0.55–0.94) |
0.019 |
|||||||||||||
History of atrial fibrillation |
0.75 (0.56–1.0) |
0.049 |
|||||||||||||
Prior myocardial infarction |
0.77 (0.61–0.98) |
0.034 |
|||||||||||||
GRACE score > 200 v < 100 |
0.94 (0.44–2.04) |
0.881 |
|||||||||||||
Age in years |
0.97 (0.96–0.98) |
< 0.001 |
|||||||||||||
Male |
1.47 (1.22–1.79) |
< 0.001 |
|||||||||||||
GRACE score 101–150 v < 100 |
1.77 (1.35–2.33) |
< 0.001 |
|||||||||||||
GRACE score 151–200 v < 100 |
1.96 (1.28–2.99) |
0.002 |
|||||||||||||
Onsite cardiac surgical service |
4.13 (2.29–7.45) |
< 0.001 |
|||||||||||||
Admission with high-risk NSTEACS |
5.10 (2.84–9.13) |
< 0.001 |
|||||||||||||
Admission with suspected STEMI |
6.31 (3.01–13.30) |
< 0.001 |
|||||||||||||
GRACE = Global Registry of Acute Coronary Events (higher score implies greater risk). NSTEACS = non-ST-segment-elevation acute coronary syndrome. STEMI = ST-segment-elevation myocardial infarction. c-index = 0.853. |
|||||||||||||||
6 Kaplan–Meier survival curves for patients discharged with a coronary diagnosis
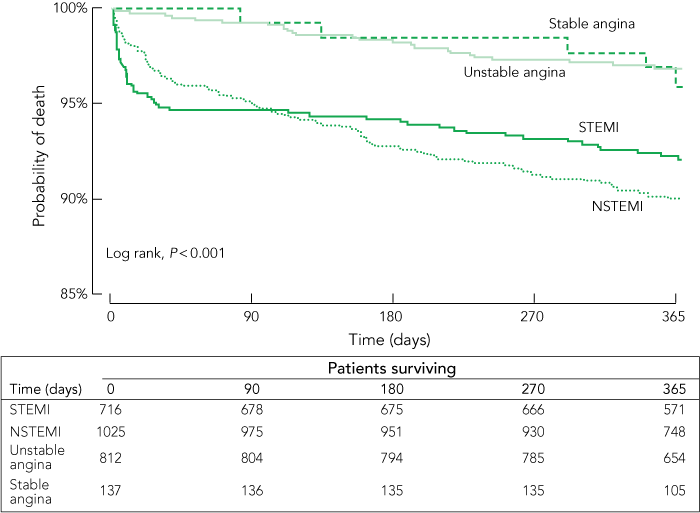
STEMI = ST-segment-elevation myocardial infarction. NSTEMI = non-ST-segment-elevation myocardial infarction. |
7 Clinical outcomes from enrolment to 12 months among patients discharged with a coronary diagnosis
Outcome |
STEMI (n = 716) |
NSTEMI (n = 1025) |
Unstable angina (n = 812) |
Stable angina (n = 137) |
P |
||||||||||
Death |
57 (8.0%) |
108 (10.5%) |
27 (3.3%) |
5 (3.6%) |
< 0.001 |
||||||||||
Myocardial infarction* |
59 (8.2%) |
127 (12.4%) |
28 (3.4%) |
3 (2.2%) |
< 0.001 |
||||||||||
Stroke |
5 (0.7%) |
6 (0.6%) |
3 (0.4%) |
1 (0.7%) |
0.835 |
||||||||||
Revascularisation† |
112 (15.6%) |
133 (13.0%) |
72 (8.9%) |
11 (8.0%) |
< 0.001 |
||||||||||
STEMI = ST-segment-elevation myocardial infarction. NSTEMI = non-ST-segment-elevation myocardial infarction. *Initial or recurrent. † Revascularisation (percutaneous coronary intervention or coronary artery bypass grafting) conducted after the index hospitalisation. |
|||||||||||||||
8 Baseline risk, and medications prescribed at discharge and persistent use at 6 months for patients with a coronary diagnosis treated invasively and conservatively
Conservative |
Invasive |
P |
|||||||||||||
No. of patients |
882 |
1785 |
|
||||||||||||
Baseline risk — median GRACE score (IQR) |
134 (105–167) |
126 (103–151) |
0.0001 |
||||||||||||
Medications at discharge |
|
|
|
||||||||||||
Aspirin |
730 (82.8%) |
1677 (94.0%) |
< 0.001 |
||||||||||||
Clopidogrel |
405 (45.9%) |
1315 (73.7%) |
< 0.001 |
||||||||||||
β-Blockers |
565 (64.1%) |
1358 (76.1%) |
< 0.001 |
||||||||||||
ACE-inhibitor or AR-antagonist |
547 (62.0%) |
1356 (76.0%) |
< 0.001 |
||||||||||||
Statin |
657 (74.5%) |
1618 (90.6%) |
< 0.001 |
||||||||||||
No. of patients surviving to 6 months |
818 |
1741 |
|
||||||||||||
Medication persistence at 6 months* |
|
|
|
||||||||||||
Aspirin |
579 (70.8%) |
1490 (85.6%) |
< 0.001 |
||||||||||||
Clopidogrel |
322 (39.4%) |
1062 (61.0%) |
< 0.001 |
||||||||||||
β-Blockers |
489 (59.8%) |
1177 (67.6%) |
< 0.001 |
||||||||||||
ACE-inhibitor or AR-antagonist |
500 (61.1%) |
1280 (73.5%) |
< 0.001 |
||||||||||||
Statin |
573 (70.0%) |
1503 (86.3%) |
< 0.001 |
||||||||||||
GRACE = Global Registry of Acute Coronary Events (higher score implies greater risk). ACE = angiotensin-converting enzyme. AR = angiotensin receptor. * Rates reported among survivors to 6 months. Values are number (%) of patients unless otherwise indicated. |
|||||||||||||||
Received 21 November 2006, accepted 28 February 2008
- Derek P Chew1,2
- John V Amerena3
- Steve G Coverdale4
- Jamie M Rankin5
- Carolyn M Astley1
- Ashish Soman6
- David B Brieger7
- on behalf of the ACACIA investigators
- 1 Flinders University, Adelaide, SA.
- 2 Flinders Medical Centre, Adelaide, SA.
- 3 Geelong Hospital, Geelong, VIC.
- 4 Nambour General Hospital, Nambour, QLD.
- 5 Royal Perth Hospital, Perth, WA.
- 6 Sanofi-Aventis Australia, Sydney, NSW.
- 7 Concord Hospital, Sydney, NSW.
The study was organised and run by the following people:
Steering committee: Derek Chew, Flinders University/Flinders Medical Centre; John Amerena, Geelong Hospital; David Brieger, Concord Hospital; Steve Coverdale, Nambour General Hospital; Jamie Rankin, Royal Perth Hospital.
Data management and analysis: Cardiovascular Outcomes Research (COR): Carolyn Astley, Danni Molloy, Sue Mattchoss, Luan Huynh.
Sponsor: Sanofi-Aventis Australia Pty Ltd.
Investigators (by state): Australian Capital Territory: Dr Tan Ren, Canberra Hospital. New South Wales: Associate Professor David Brieger, Concord Hospital; Associate Professor MA Fitzpatrick, Nepean Hospital; Professor Peter Fletcher, John Hunter Hospital; Dr David Rees, St George Hospital; Dr Craig Juergens, Liverpool Hospital; Dr Jonathon Waites, Coffs Harbour Hospital; Dr Greg Nelson, Royal North Shore Hospital; Dr Michael Sinclair, Dubbo Base Hospital. Victoria: Dr John Amerena, Geelong Hospital; Professor Yean Lim, Western Hospital; Dr Mark Horrigan, Austin Health; Dr Leeanne Grigg, Royal Melbourne Hospital; Dr David Eccleston, Northern Hospital Clinical Trials Unit; Dr Greg Szto, Peninsula Private Hospital; Associate Professor Gishel New, Box Hill Hospital; Dr Christopher Medley, Wodonga Regional Health Service. Queensland: Dr Steve Coverdale, Nambour General Hospital; Professor Laurie Howes, Gold Coast Hospital; Dr David Cross, Wesley Hospital; Dr Paul Garrahy, Princess Alexandra Hospital; Dr Darren Walters, Prince Charles Hospital; Dr Spencer Toombes, Toowoomba Health Services; Dr Prasad Challa, Cairns Base Hospital; Dr Kumar Gunawardane, Townsville Hospital; Dr William Parsonage, Royal Brisbane Hospital; Dr Raj Shetty, Rockhampton Hospital. South Australia: Professor Derek Chew, Flinders Medical Centre; Professor Stephen Worthley, Royal Adelaide Hospital; Professor John Horowitz, Queen Elizabeth Hospital; Dr Samuel Varughese, Mt Gambier Hospital; Dr Christopher Zeitz, Port Augusta Hospital; Dr Margaret Arstall, Lyell McEwin Hospital. Western Australia: Dr Jamie Rankin, Royal Perth Hospital; Dr Barry McKeown, Fremantle Hospital; Dr Johan Janssen, Kalgoorlie Regional Hospital; Associate Professor Joseph Hung, Sir Charles Gairdner Hospital. Tasmania: Dr Philip Roberts-Thomson, Royal Hobart Hospital. Northern Territory: Dr Marcus Iton, Darwin Private Hospital; Dr Alex Brown, Menzies School of Health Research.
The study was sponsored by Sanofi-Aventis Australia. Analysis of data and writing of manuscript were conducted independently of the sponsor. The sponsor had rights to review the manuscript, but no response was made.
- 1. Bavry AA, Kumbhani DJ, Rassi AN, et al. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48: 1319-1325.
- 2. Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006; 367: 579-588.
- 3. Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol 2007; 49: 422-430.
- 4. Guidelines for the management of acute coronary syndromes 2006. Med J Aust 2006; 184 (8 Suppl): S9-S29. <MJA full text>
- 5. Chew DP, Allan RM, Aroney CN, Sheerin NJ. National data elements for the clinical management of acute coronary syndromes. Med J Aust 2005; 182 (9 Suppl): S1-S14. <MJA full text>
- 6. Chew DP, Amerena J, Coverdale S, et al. Current management of acute coronary syndromes in Australia: observations from the acute coronary syndromes prospective audit. Intern Med J 2007; 37: 741-748.
- 7. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265-2281.
- 8. Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA 2007; 297: 1892-1900.
- 9. Montalescot G, Dallongeville J, Van Belle E, et al. STEMI and NSTEMI: are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry). Eur Heart J 2007; 28: 1409-1417.
- 10. Bhatt DL, Roe MT, Peterson ED, et al. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 2004; 292: 2096-2104.
- 11. Roe MT, Peterson ED, Newby LK, et al. The influence of risk status on guideline adherence for patients with non-ST-segment elevation acute coronary syndromes. Am Heart J 2006; 151: 1205-1213.
- 12. Yan AT, Yan RT, Tan M, et al. In-hospital revascularization and one-year outcome of acute coronary syndrome patients stratified by the GRACE risk score. Am J Cardiol 2005; 96: 913-916.
- 13. Scott IA, Derhy PH, O’Kane D, et al. Discordance between level of risk and intensity of evidence-based treatment in patients with acute coronary syndromes. Med J Aust 2007; 187: 153-159. <MJA full text>
- 14. Steg PG, Lopez-Sendon J, Lopez de Sa E, et al. External validity of clinical trials in acute myocardial infarction. Arch Intern Med 2007; 167: 68-73.
- 15. Kandzari DE, Roe MT, Chen AY, et al. Influence of clinical trial enrollment on the quality of care and outcomes for patients with non-ST-segment elevation acute coronary syndromes. Am Heart J 2005; 149: 474-481.





Abstract
Objective: To describe the impact of invasive management on 12-month survival among patients with suspected acute coronary syndrome (ACS) in Australia.
Design and setting: Prospective nationwide multicentre registry.
Patients: Patients presenting to 24 metropolitan and 15 non-metropolitan hospitals with ST-segment-elevation myocardial infarction (STEMI), and high-risk and intermediate-risk non-ST-segment-elevation ACS (NSTEACS) between 1 November 2005 and 31 July 2007.
Main outcome measures: Death, myocardial infarction (MI) or recurrent MI, revascularisation and stroke at 12 months.
Results: Among 3402 patients originally enrolled, vital status at 12 months was available for 3393 (99.7%). Patients from non-metropolitan areas (810) constituted 23.9% of patients. Early invasive management was more commonly undertaken among patients with STEMI (STEMI, 89.7% v non-STEMI, 70.8% v unstable angina, 44.8% v stable angina, 35.8%; P < 0.001). Factors most associated with receiving invasive management included admission with suspected STEMI or high-risk NSTEACS, being male and the hospital having an onsite cardiac surgical service. Overall mortality by 12 months among patients with STEMI, non-STEMI, unstable angina and stable angina was 8.0%, 10.5%, 3.3%, and 3.7% (P < 0.001), respectively. After adjusting for a propensity model predicting early invasive management and other known confounders, early invasive management was associated with a 12-month mortality hazard ratio of 0.53 (95% CI, 0.34–0.84, P = 0.007).
Conclusions: A substantial burden of late morbidity and mortality persists among patients with ACS within contemporary Australian clinical practice. Under-use of invasive management may be associated with an excess in 12-month mortality, suggesting the need for more use of invasive management among these patients.