Australia currently has one of the lowest rates of daily smoking in the world thanks to high taxation, comprehensive advertising bans, restrictions on smoking in public places and workplaces, and funding for smoking cessation programs. Nonetheless, 2.9 million Australians were daily smokers in 2004,1 and tobacco still accounted for 8% of disease burden in 2003.2 Even if the prevalence of smoking continues to fall in Australia, at current rates it will still be many years before tobacco smoking disappears as a public health issue.
The key to reducing the health burden of tobacco in the medium term is increasing cessation among smokers. The problem is that only about 5% of smokers who try to quit unassisted achieve long-term abstinence in any year,3 and available cessation aids such as nicotine replacement and bupropion at best double or treble this quit rate and have had low uptake in the community.4
A neglected aim of Australia’s National Tobacco Strategy 2004–2009 is “to reduce the harm associated with continuing use of, and dependence on, tobacco and nicotine”. On the available evidence, Swedish snus (low nitrosamine, moist oral snuff) appears to be a good candidate for achieving this aim. As a smokeless tobacco (SLT) product, snus does not produce any of the combustion products of smoking and it is manufactured in a way that produces low levels of tobacco-specific nitrosamines, the main carcinogens responsible for oral cancers in users of other SLT products.5 Studies of Swedish men who have used snus for 20 years have failed to find an increased risk of oral cancers or cardiovascular disease.6-8 Despite the high prevalence of snus use (21% of men are daily snus users), tobacco-related mortality in Sweden is among the lowest in the developed world.9,10
The increased use of snus in Sweden over the past 20 years has coincided with substantial reductions in smoking prevalence and tobacco-related mortality.11 Although Sweden’s tobacco control policies have undoubtedly contributed to this decline, the popularity of snus has also played a role, because the decline in daily smoking prevalence has been greater among males (from 40% in 1976 to 15% in 2002) than females (34% in 1976 to 20% in 2002). Male snus use rose from 10% in 1976 to 23% in 2002, while only 2% of Swedish women used snus in 2002.7,10 The Swedish population prevalence of tobacco use has remained relatively steady at around 40%, but 58% of daily tobacco users now use snus instead of smoking.10 Many former smokers in Sweden have quit through using snus,7,10,12,13 suggesting it may be a more effective cessation aid, and a more attractive long-term alternative to cigarettes, than pharmaceutical nicotine because its nicotine delivery and social aspects are similar to those of smoking.
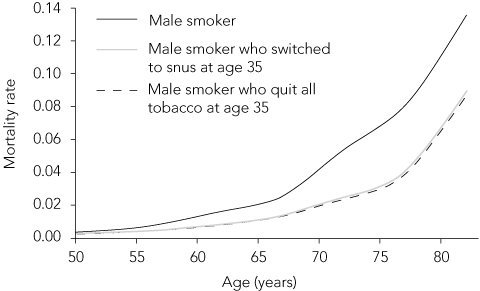
Snus use may increase the risk of pancreatic cancer,14 and there remains the possibility of some residual risk of cardiovascular disease from the direct effects of nicotine.15 But epidemiological studies suggest that any such health risks of snus will still be much lower than those of smoking,16,17 and probably less than those of conventional SLT because of the lower nitrosamine content of snus.7,18 Recent epidemiological modelling we have done19 also indicates that there are only small differences in mortality between smokers who quit and those who switch to snus (Box).
In Australia, the most dangerous tobacco products (cigarettes) are the least regulated, while oral tobacco products, including snus, cannot be legally sold because in 1991 the federal government banned the manufacture, importation and commercial supply of chewing tobacco and oral snuff in Australia under the Trade Practices Act 1974 (Cwlth). Individuals are permitted to import SLT for personal use without a permit in amounts up to 1.5 kg,20 but a recent increase in the taxation on SLT from $2.30/kg to $300.39/kg has made importation of these products prohibitively expensive.21
The levels of carcinogens and other toxins in smoked tobacco products are currently unregulated, and tobacco manufacturers can introduce new tobacco products into Australia so long as they are smoked. For example, the federal government publicly welcomed the introduction of a new form of cigarette by Philip Morris that makes implied claims of lower health risks than traditional cigarettes22,23 in the absence of any supporting evidence.
Public health authorities in Australia and the United States have also claimed that SLT products: “are just as bad for your health as cigarettes”.24 The epidemiological evidence shows that this is untrue. Dissemination by governments of misinformation on the relative harms of snus creates scepticism and mistrust of public health messages.25 It is paternalistic to misinform smokers about the risks of SLT products for fear of increasing population nicotine use.25 We think it is also unethical to deny smokers access to a product that may reduce their health risk while cigarettes are readily available and very few quit attempts succeed.3
Public health legislation should be reviewed when new evidence contradicts its underlying assumptions. The original case for the federal ban on commercial supply of SLT was based on its high nitrosamine content and epidemiological evidence “of a significant positive association between the use of oral snuffs and the development of oral cancer”.26 As this is not true of low nitrosamine SLT, the ban on these products should be reconsidered.
Snus needs to be regulated in ways that address a number of legitimate concerns that have been raised. One concern is that promoting snus use may reduce overall tobacco-related mortality and morbidity among smokers who switch, but at the cost of increasing tobacco-related disease in the non-smoking population. We think this unlikely, because epidemiological modelling suggests that the health gain from one would-be smoker who uses snus instead of cigarettes would only be offset if 17 non-smokers who would not otherwise have smoked started to use snus and did so for the rest of their lives.19 If the goal of tobacco control is to reduce tobacco-related disease, rather than tobacco use per se, then allowing inveterate smokers to use snus looks a promising public health policy.
A second concern is that increasing snus use would reduce rates of smoking cessation. That is, smokers who may otherwise quit because of the inconvenience of smoking bans may use snus when smoking is not allowed and smoke when it is. In Sweden, however, smoking prevalence and tobacco-related mortality have both declined as snus use has increased. This is because people who start to use snus are much less likely to start smoking than those who do not use snus, and smokers who start using snus are more likely to quit than those who do not.10 Dual use of snus and cigarettes is uncommon in Sweden, and appears to be a “transitional state” to abstinence or continued snus use.12 Dual use of snus could allow some smokers to continue smoking in the face of public smoking bans, but there is nothing stopping addicted smokers using nicotine-replacement therapy in the same way or simply going outside to smoke.
A third concern is that tobacco companies may use lower risk tobacco products to undermine tobacco advertising bans.27 However, the need to inform smokers of the reduced risk of snus does not require tobacco companies to advertise these products. Rather, factual information and advice could be provided via QUIT lines, general medical practitioners and pharmacists, who could recommend snus use as an additional path to quitting, or as a second-best option to quitting. Mandatory warning labels on snus packs could also advise of the probable health gains and risks of snus (eg, oral and pancreatic cancers and possibly cardiovascular disease).
Australia’s existing regulatory situation allows these concerns about snus promotion to be addressed. Australia’s ban on advertising tobacco products28 prevents tobacco companies from promoting the dual use of SLT with cigarettes. As a new product, the nitrosamine content of SLT products could also be controlled by specifying maximum permissible levels of carcinogens. We could also reduce the attractiveness of these products to non-tobacco users (eg, children) by banning the importation and sale of flavoured snus.
A fourth objection is that snus will not be an attractive alternative to cigarettes because Australians have never had a tradition of SLT use.15 If these critics are correct, then no Australians will use snus and relaxing the ban will have no effect. We think it unlikely that no Australian smokers will be interested in snus, as the market for SLT products appears to be growing in both the US (oral snuff) and the United Kingdom (nasal snuff). Insisting on a continuation of the current ban on SLT will prevent this claim from ever being tested.
We should reduce the absurdly high customs tax on SLT products to make snus more affordable and easier to import.
We should allow SLT products that comply with set standards of carcinogens to be sold under the counter in a limited range of licensed outlets (eg, tobacconists or pharmacies). Sales should be monitored to assess smokers’ interest in these products.
If smokers do use these products, we should allow SLT products to compete with smoked tobacco under the same restrictive conditions of sale, but with lower rates of taxation on SLT products.
Medical practitioners and QUIT lines could encourage inveterate smokers to switch to SLT as a way of reducing the harm caused by their tobacco use.
Snus, or other forms of SLT or “clean nicotine” products may never completely replace the cigarette, but tobacco smokers who switch to snus will substantially reduce the health risks of their tobacco use. As the Royal College of Physicians has recently concluded,29 there is a strong case on public health and ethical grounds for allowing inveterate smokers who want to reduce their health risks to have access to snus. This could be achieved immediately by reducing the prohibitive import duty on SLT to make importation for personal use more affordable. Legislation is also needed: to reverse the Australian ban on the commercial importation and supply of low nitrosamine SLT; and to allow its sale under restrictive conditions that address the legitimate concerns raised about the promotion of these products to non-smokers.
- Coral E Gartner1
- Wayne D Hall2
- School of Population Health, University of Queensland, Brisbane, QLD.
Neither author has had any past connection, and neither has any present connection, with the tobacco industry. We have never accepted funds from a tobacco company, including manufacturers of snus or other smokeless tobacco products.
- 1. Australian Institute of Health and Welfare. 2004 national drug strategy household survey: detailed findings. Drug Statistics Series No. 16. Canberra: AIHW, 2005. (AIHW Cat. No. PHE 66.)
- 2. Begg S, Vos T, Goss J, et al. The burden of disease and injury in Australia 2003. Canberra: AIHW, 2007. (AIHW Cat. No. PHE 82.)
- 3. Fiore MC, Novotny TE, Pierce JP, et al. Methods used to quit smoking in the United States. Do cessation programs help? JAMA 1990; 263: 2760-2765.
- 4. Cummings KM, Hyland A. Impact of nicotine replacement therapy on smoking behavior. Annu Rev Public Health 2005; 26: 583-599.
- 5. Österdahl B-G, Jansson C, Paccou A. Decreased levels of tobacco-specific N-nitrosamines in moist snuff on the Swedish market. J Agric Food Chem 2004; 52: 5085-5088.
- 6. Asplund K. Smokeless tobacco and cardiovascular disease. Prog Cardiovasc Dis 2003; 45: 383-394.
- 7. Foulds J, Ramström L, Burke M, et al. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob Control 2003; 12: 349-359.
- 8. Critchley JA, Unal B. Health effects associated with smokeless tobacco: a systematic review. Thorax 2003; 58: 435-443.
- 9. Peto R, Lopez AD, Boreham J, Thun M. Mortality from smoking in developed countries 1950–2000: Sweden. 2nd ed, revised June 2006. http://www.deathsfromsmoking.net (accessed Oct 2007).
- 10. Ramström LM, Foulds J. Role of snus in initiation and cessation of tobacco smoking in Sweden. Tob Control 2006; 15: 210-214.
- 11. Bates C, Fagerström K, Jarvis MJ, et al. European Union policy on smokeless tobacco: a statement in favour of evidence based regulation for public health. Tob Control 2003; 12: 360-367.
- 12. Furberg H, Lichtenstein P, Pedersen NL, et al. Cigarettes and oral snuff use in Sweden: prevalence and transitions. Addiction 2006; 101: 1509-1515.
- 13. Gilljam H, Galanti MR. Role of snus (oral moist snuff) in smoking cessation and smoking reduction in Sweden. Addiction 2003; 98: 1183-1189.
- 14. Luo J, Ye W, Zendehdel K, et al. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet 2007; 369: 2015-2020.
- 15. Broadstock M. Systematic review of the health effects of modified smokeless tobacco products. NZHTA Report 2007; 10 (1).
- 16. Roth HD, Roth AB, Liu X. Health risks of smoking compared with Swedish snus. Inhal Toxicol 2005; 17: 741-748.
- 17. Rodu B, Jansson C. Smokeless tobacco and oral cancer: a review of the risks and determinants. Crit Rev Oral Biol Med 2004; 15: 252-263.
- 18. Ramström LM. Snuff — an alternative nicotine delivery system. In: Ferrence R, Slade J, Room R, Pope M, editors. Nicotine and public health. Washington, DC: American Public Health Association, 2000.
- 19. Gartner CE, Hall WD, Vos T, et al. Assessment of Swedish snus for tobacco harm reduction: an epidemiological modelling study. Lancet 2007; 369: 2010-2014.
- 20. Customs (Prohibited Imports) Regulations 1956 (Cwlth).
- 21. Excise Tariff Amendment (Fuel Tax Reform and Other Measures) Act 2006 (Cwlth).
- 22. Pyne C. Harm reduction a positive step [media release]. Secretary to Minister for Health and Ageing. 24 Mar 2005. http://www.health.gov.au/internet/ministers/publishing.nsf/Content/health-mediarel-yr2005-cp-pyn014.htm?OpenDocument&yr=2005&mth=3 (accessed Oct 2007).
- 23. Houston C. Electronic smokes get go-ahead. The Age (Melbourne) 2007; 7 Jul: 9.
- 24. Robertson S. New laws to snuff out smokeless tobacco products [media release]. Minister for Health, Queensland. 2006; 11 Oct. http://statements.cabinet.qld.gov.au/MMS/StatementDisplaySingle.aspx?id=48421 (accessed Oct 2007).
- 25. Phillips CV, Wang C, Guenzel B. You might as well smoke: the misleading and harmful public message about smokeless tobacco. BMC Public Health 2005; 5: 31.
- 26. Tamblyn J. The TPC recommends restrictions on ‘smokeless tobacco’ [media release]. Office of the First Assistant Commissioner, Trade Practices Commission. 1989; 25 Sep. Cited in: Winstanley M, Woodward S, et al. Tobacco in Australia: facts and issues. 2nd ed. Melbourne: Quit Victoria, 1995.
- 27. Chapman S. Public health advocacy and tobacco control: making smoking history. Oxford: Blackwells, 2007; Chapter 4.
- 28. Tobacco Advertising Prohibition Act 1992 (Cwlth).
- 29. Royal College of Physicians. Harm reduction in nicotine addiction: helping people who can’t quit. A report by the Tobacco Advisory Group of Royal College of Physicians. London: RCP, 2007. http://www.rcplondon.ac.uk/pubs/brochure.aspx?e=234 (accessed Oct 2007).





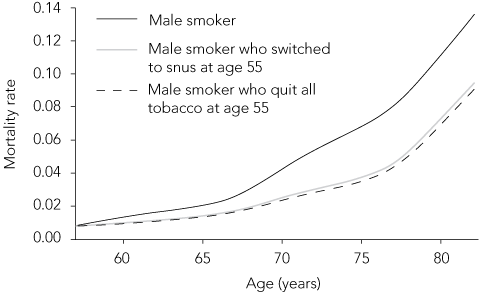
Abstract
In Australia, 2.9 million people continue to smoke daily, and tobacco still accounts for 8% of disease burden.
Tobacco harm-reduction strategies, such as the use of Swedish snus, have been suggested as a way to further reduce this disease burden.
In Australia, the most dangerous tobacco products (cigarettes) are the least regulated, while oral tobacco products, including snus, cannot be sold legally.
Recent epidemiological modelling indicates that there are only small differences in life expectancy between smokers who quit and those who switch to snus.
There is a case on public health and ethical grounds for allowing inveterate smokers who want to reduce their health risks to access snus.
At a minimum, the recent increase in tax on smokeless tobacco should be reversed, and the ban on the commercial importation and supply of low nitrosamine smokeless tobacco should be reconsidered in light of the epidemiological evidence on its potential to reduce tobacco-related disease in smokers.