The Australasian Creatinine Consensus Working Group recommended in June 2005 that Australasian laboratories automatically report an estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) formula each time a serum creatinine test was requested.1 Survey findings and anecdotal information indicate that the vast majority of laboratory reports now include an eGFR. In addition, standardisation of μmol/L as the unit for serum creatinine concentration has largely been achieved. The response of clinicians to the introduction of eGFR has been strongly positive, with easier identification of chronic kidney disease, better decision making for affected patients, and more appropriate referral patterns being identified as outcomes.
This revised statement should be read in conjunction with the original consensus statement (see Box 1 for a summary of the principal changes).1 The recommendations in this document are in addition to and do not replace the original recommendations.
Since the original MDRD article2 was published, there has been considerable focus on the variability in results derived from assays in routine use for measuring serum creatinine concentration. An international process of assay standardisation has been undertaken, led by the National Kidney Disease Education Program in the United States and supported by other national and international bodies.3,4 Isotope dilution mass spectrometry (IDMS) has been accepted as the reference method, and diagnostic companies are producing revised assays to give results aligned to this method. As most assays in routine use in Australasia are variations of the non-specific Jaffe reaction,5 this generally involves an adjustment for the expected effects of non-creatinine chromogens in an average sample. The results from IDMS-aligned assays are different from those derived from the assay used to establish the MDRD equation, and so a revised version of the equation has been published, known as the “175” formula:
The revised MDRD formula (the “175” formula)6
eGFR = 175 × (SCR × 0.0113)–1.154 × (age)–0.203 × (0.742 [if female])
where MDRD = Modification of Diet in Renal Disease,2 eGFR = estimated glomerular filtration rate (mL/min/1.73 m2), SCR = serum creatinine concentration (μmol/L), and age is expressed in years.
The Working Group has validated the revised equation using data from two local centres and recommends the introduction of this formula for laboratories using assays aligned to the IDMS international standard. Peake and Whiting5 have evaluated the assays in common use in Australia against the reference preparation SRM 967 and shown that the majority of assays are acceptable by comparison with this standard. Revised versions of other assays are expected in 2007.
It is important to recognise that improvements in the trueness of assays for serum creatinine do not reduce the potential for assay interference. Peake and Whiting5 have demonstrated the effects of common interfering substances such as albumin, glucose, pyruvate, bilirubin, haemoglobin F and cephalosporins (especially cefpirome) on a commonly used Jaffe creatinine assay, but the interferences are likely to be different for each manufacturer. Where interference is considered to be likely, the use of an enzymatic method may be better. Some biological influences on creatinine are not assay-related — for example, the effect of a cooked meat meal, which may cause a temporary increase of over 20 μmol/L in measured creatinine concentration that can last several hours.7
The original recommendation, similar to that adopted in the United States, that eGFR values greater than 60 mL/min/1.73 m2 should be reported as “> 60 mL/min/1.73 m2” rather than as a numerical result was based on evidence available at the time (summarised in the original statement1). After considerable discussion, the Working Group agreed to recommend that Australasia now adopt the United Kingdom’s approach,8 which is for laboratories to report numerical values up to 90 mL/min/1.73 m2, with results of over 90 mL/min/1.73 m2 to be reported as “> 90 mL/min/1.73 m2”.
This decision took the following factors into account:
There were some clinical situations in which knowing specific eGFR values in the range 60–90 mL/min/1.73 m2 would be useful — for example, in providing earlier warning of declining eGFR and allowing trends over time to be monitored.
The relative accuracy of eGFR compared with direct measurement of GFR was similar throughout the range 0–90 mL/min/1.73 m2.
Standardised creatinine assays would be likely to show better agreement with each other in the eGFR range 60–90 mL/min/1.73 m2 than was the case with older assays.
Advice would be offered to clinicians that further investigation was usually only required if the eGFR fell to < 60 mL/min/1.73 m2. Clinical judgement could determine whether an eGFR in the range 60–90 mL/min/1.73 m2 in a young adult required investigation.
A substantial number of laboratories in some Australian states had already begun to report specific eGFR values up to 90 mL/min/1.73 m2, which may have been a source of confusion and uncertainty among medical practitioners.
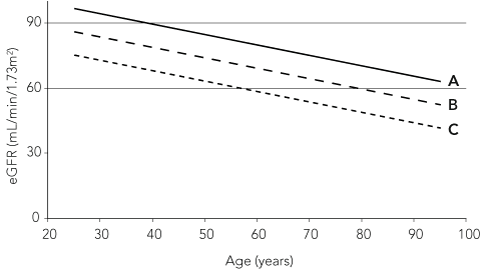
While it is commonly stated that the “normal range” for eGFR is > 90 mL/min/1.73 m2, this does not reflect the distribution of eGFR values in the Australian community presenting to medical practitioners (Box 2). By the age of 40 years, the median eGFR in the Australian community is about 90 mL/min/1.73 m2, and by the age of 80 years, the median value is 70 mL/min/1.73 m2. Thus, well over 50% of Australians aged over 40 years will have an eGFR of < 90 mL/min/1.73 m2.
Age-related decision points have not been agreed for eGFR,9 and the UK guidelines specifically state that “age-related reference intervals are not recommended”.8 While most studies show that GFR declines with age (Box 2), accepting this as normal runs the risk of “normalising” a pathological state caused by age-related diseases rather than by age itself. A fall in GFR is not an inevitable consequence of ageing: the Baltimore Longitudinal Study on Ageing showed that the decline in GFR with age is largely the result of hypertension.10 Although the impact of reduced GFR seems largely independent of age, one large study has demonstrated a weaker association between reduced GFR and mortality in people aged ≥ 65 years than in younger people.11
The original MDRD formula contains a factor to be applied to African Americans, raising the possibility that other variations in the formula may be required for optimal performance in different ethnic groups. The publication of our initial consensus statement was interpreted by some to discourage the use of eGFR (MDRD) in Aboriginal and Torres Strait Islander peoples and led to correspondence in this Journal.12 Our intention had simply been to highlight that in certain populations the MDRD formula for estimating GFR had not been validated, and that in those populations caution should be exercised in its application. To date, there have been no studies validating the use of the MDRD formula in any specific non-Caucasian groups in Australia or New Zealand.
The original consensus statement noted that drug-dosing adjustments for patients with reduced kidney function are currently based mainly on creatinine clearance determination or the Cockcroft–Gault formula and that these results may differ significantly from the eGFR. Attention was also drawn to the eGFR value being corrected for body surface area and thus needing to be “uncorrected” if an actual GFR value was required for drug-dosing decisions in people of large or small body size. This cautious approach appeared justified at the time and has been emphasised in a recent publication.13 A stricter approach has been taken in the Australian medicines handbook: “Be aware that the estimate of glomerular filtration rate (eGFR) automatically reported with electrolyte test results is not appropriate for use in dosage calculations.”14 As new drugs are introduced into clinical practice, we can expect to see a progressive increase in the use of eGFR as a guide to dose adjustment for patients with CKD. In the interim, the question arises as to whether it is appropriate to use eGFR for dose adjustment.
In most clinical situations (particularly in general practice), the prescriber is unaware of the patient’s kidney function. Few general practitioners or specialists routinely calculate the GFR using the Cockcroft–Gault formula before prescribing. The availability of an eGFR value on a general chemistry report has increased the frequency with which practitioners have access to a measure of GFR before prescribing.
The published recommendations are potentially confusing, with variation in terminology of kidney dysfunction and different decision making points for the same drug.15
1 Comparison between original and revised recommendations: summary
An eGFR shall be calculated and reported with every request for serum creatinine concentration |
|||||||||||||||
Automatic reporting of eGFR may include age-related reference intervals for people aged ≥ 65 years |
|||||||||||||||
- Timothy H Mathew1
- David W Johnson2,3
- Graham R D Jones4,5
- on behalf of the Australasian Creatinine Consensus Working Group
- on behalf of the Australasian Creatinine Consensus Working Group
- 1 Kidney Health Australia, Adelaide, SA.
- 2 Princess Alexandra Hospital, Brisbane, QLD.
- 3 University of Queensland, Brisbane, QLD.
- 4 SydPath, St Vincent’s Hospital, Sydney, NSW.
- 5 University of New South Wales, Sydney, NSW.
None identified.
- 1. Australasian Creatinine Consensus Working Group. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust 2005; 183: 138-141. <MJA full text>
- 2. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461-470.
- 3. Myers GL, Miller GW, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5-18.
- 4. National Kidney Disease Education Program. Creatinine Standardization Program. http://www.nkdep.nih.gov/labprofessionals/index.htm (accessed Apr 2007).
- 5. Peake M, Whiting M. Measurement of serum creatinine — current status and future goals. Clin Biochem Rev 2006; 27: 173-184.
- 6. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247-254.
- 7. Preiss DJ, Godber IM, Lamb EJ, et al. The influence of a cooked-meat meal on estimated glomerular filtration rate. Ann Clin Biochem 2007; 44: 35-42.
- 8. Joint Specialty Committee on Renal Medicine of the Royal College of Physicians and the Renal Association, and the Royal College of General Practitioners. Chronic kidney disease in adults: UK guidelines for identification, management and referral. London: Royal College of Physicians, 2006. http://www.bgs.org.uk/Publications/Publication%20Downloads/RCPKidneyOrderForm.pdf (accessed Aug 2007).
- 9. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39 (2 Suppl 1): S1-S246.
- 10. Lindeman RD, Tobin JD, Shock NW. Association between blood pressure and the rate of decline in renal function with age. Kidney Int 1984; 26: 861-868.
- 11. O’Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol 2006; 17: 846-853.
- 12. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate [matters arising]. Med J Aust 2007; 184: 41-43. <MJA full text>
- 13. Faull R. Prescribing in renal disease. Aust Prescr 2007; 30: 17-20.
- 14. Prescribing in renal impairment. In: Bochner F, Buckley N, Del Mar C, et al, editors. Australian medicines handbook. Adelaide: AMH, 2007. http://amh.hcn.net.au (accessed Sep 2007).
- 15. Vidal L, Shavit M, Fraser A, et al. Systematic comparison of four sources of drug information regarding adjustment of dose for renal function. BMJ 2005; 331: 263-266.





Abstract
Since publication of the Australasian Creatinine Consensus Working Group’s position statement in 2005, most Australasian laboratories now automatically report an estimated glomerular filtration rate (eGFR) (based on the Modification of Diet in Renal Disease [MDRD] formula) with results of serum creatinine tests in adults.
Anecdotal evidence suggests that automatic reporting of eGFR helps to identify asymptomatic kidney dysfunction at an earlier stage and to develop rational and appropriate management plans.
Changes to the measurement and calibration of serum creatinine assays and issues regarding implementation of eGFR in clinical practice led the Australasian Creatinine Consensus Working Group to reconvene in 2007.
The recommendations contained here build on the original 2005 position statement and consolidate the role of eGFR in clinical practice.
The Working Group recommends that the eGFR upper reporting limit be extended to 90 mL/min/1.73 m2, with eGFR values above this amount being reported as “> 90 mL/min/1.73 m2”, rather than as a precise figure.
The Working Group has concluded that it is currently premature to recommend age-related decision points for eGFR. However, it is appropriate to advise medical practitioners that, in people aged ≥ 70 years, an eGFR in the range 45–59 mL/min/1.73 m2, if stable over time and unaccompanied by other evidence of kidney damage, may be interpreted as consistent with a typical eGFR for this age group and is unlikely to be associated with chronic kidney disease-related complications.
Pending publication of validation studies, the Working Group recommends that Australasian laboratories continue to automatically report eGFR in Aboriginal and Torres Strait Islander peoples and other ethnic groups.
The Working Group supports the use of eGFR to assist drug dosing decision making in general practice.