Clinical record
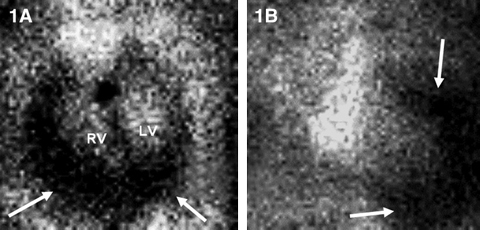
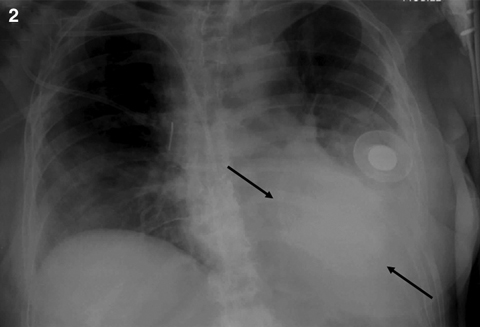
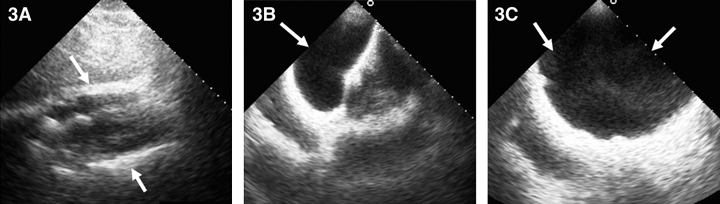
On the GHPS (Figure 1) a radiolucent pericardial halo was apparent, raising concerns for pericardial effusion. The rest of the study was unremarkable, with normal regional wall motion and quantitative ejection fraction of the cardiac ventricles. Comparison with a recent chest x-ray (Figure 2) revealed radiolucency external to the chest wall. Subsequent transthoracic echocardiography (Figure 3) ruled out the presence of pericardial effusion.
|
1: GHPS left anterior oblique 45° (1A) and anterior (1B) views. Pericardial halo suggestive of pericardial effusion (arrows). |
|
2: Anteriorposterior chest x-ray showing the saline-filled breast implant as a radio-opaque shadow on the left anterior chest wall (arrows). |
|
3: Two-dimensional echocardiogram. 3A: Subcostal views showing pericardial border anteriorly and posteriorly (arrows) without evidence of pericardial effusion. 3B: Subcostal view with the transducer directed anteriorly to image the saline-filled breast implant (arrow). 3C: Anterior chest wall imaging depicting the implant (arrows). |
Current national surveys estimate that up to 4 million women, or more than 3% of the adult female population in the United States, have breast implants.1 Of clinical importance, more than 35 000 women each year received breast implant surgery for reconstruction after a mastectomy,1 and most of these patients would have one or more gated heart pool scan (GHPS) studies for the assessment of cardiac function before chemotherapy.
In Australia, the incidence of breast cancer has been steadily increasing since the early 1980s, and this is now the most common cancer among women. One in eight women will be diagnosed with breast cancer by the age of 85 years, with more than 13 000 new cases in 2006. In part because of improved and more aggressive treatment strategies, survival prospects following diagnosis continue to improve.2 The accompanying rise in the use of adjuvant chemotherapy, including the anthracyclines, has brought about a concomitant increase in the use of GHPS for monitoring drug-induced cardiac toxicity.3
Lessons from practice
Gated heart pool scan (GHPS) studies are commonly used for monitoring drug-induced cardiac toxicity in cancer patients.
Many female cancer patients will have breast implants, and these can mimic pericardial effusion on GHPS.
Clinical input, chest x-ray and, if necessary, other imaging modalities such as two-dimensional echocardiography, computed tomography or magnetic resonance imaging, are prudent for confirmation of pericardial effusion on GHPS.
GHPS was among the earliest imaging tools to be used for the diagnosis of pericardial effusion.2-7 In 1958, Rejali and colleagues,4 after observing the disparity in the size of the cardiac silhouette on radionuclide ventriculography and the chest x-rays, reported the clinical efficacy of radionuclide ventriculography in detecting pericardial effusion. Since the mid 1970s, because of its inherently higher spatial resolution, two-dimensional echocardiography has been the technique of choice for diagnosing pericardial disease in general and pericardial effusion in particular.8 Nevertheless, GHPS remains effective for diagnosing moderate to large pericardial effusion. A report in 1987 of 154 patients documented a high degree of sensitivity (100% for large and 55% for moderate to large effusions) and specificity (98%) by GHPS for diagnosing pericardial effusions.7
False-positive results may be due to benign and sinister aetiologies. Although some researchers have reported subepicardial fat as the most common cause of a false-positive finding of pericardial effusion by GHPS,9 others have documented mediastinal tumours, left ventricular hypertrophy or blood clot as some of the pathologies mimicking pericardial effusion on the GHPS.10,11 The high (98%) specificity previously reported used a set of criteria for moderate to large pericardial effusion,7 including the need for clear perceptibility of pericardial halo around all visible cardiac chambers in both the anterior and 45° left anterior oblique views of the GHPS. In the cancer population, however, the test specificity is anticipated to be lower because distortion and loculation of pericardial effusion is not uncommon.12 Test specificity is likely to be further degraded by the increasing number of women worldwide receiving breast implants for both cosmetic and postsurgical reconstruction purposes.
Of clinical importance, extracardiac and intrathoracic pathologies, including pericardial effusion, found on GHPS by and large do not impede the interpretation of cardiac function (which is the principal indication for performing the test), and the incidental findings might even provide relevant diagnostic or prognostic information.13,14
Our case highlights, particularly among female cancer patients, the need for vigilance in assessment of GHPS with extracardiac findings suggestive of pericardial effusion, with breast implant posing as a differential diagnosis of potential false-positive findings. The use of clinical input, chest x-ray and, if necessary, other imaging modalities such as two-dimensional echocardiography, computed tomography or magnetic resonance imaging9 is prudent for confirmation of pericardial effusion on GHPS.
- 1. American Society for Aesthetic Plastic Surgery. 2003 ASAPS statistics. New York: ASAPS, 2004.
- 2. Australian Institute of Health and Welfare and National Breast Cancer Centre. Breast cancer in Australia: an overview, 2006. Cancer series no. 34. Canberra: AIHW, 2006. (AIHW Cat. No. CAN 29.)
- 3. Youssef G, Links M. The prevention and management of cardiovascular complications of chemotherapy in patients with cancer. Therapy in practice. Am J Cardiovasc Drugs 2005; 5: 233-243.
- 4. Rejali AM, Macintyre WJ, Friedell HL. A radioisotope method of visualisation of blood pools. Am J Roentgenol Radium Ther Nucl Med 1958; 79; 129-137.
- 5. Christensen EE, Bonte FJ. The relative accuracy of echocardiography, intravenous CO2, and blood pool scanning in detecting pericardial effusion in dogs. Radiology 1965; 91: 265-270.
- 6. Kriss JP. Diagnosis of pericardial effusion by radioisotope angiocardiography. J Nucl Med 1969; 10: 233-241.
- 7. Rothendler JA, Edger CS, Leppo J, et al. Diagnosis of pericardial effusion from routine gated blood pool imaging. J Nucl Med 1987; 28: 1419-1423.
- 8. Weyman AE. Principles and practice of echocardiography. 2nd ed. Philadelphia: Lea & Febiger, 1994.
- 9. Kiat H, Berman DS, Maddahi J. Subepicardial fat mimicking pericardial effusion on radionuclide ventriculography: diagnosis by magnetic resonance imaging. Am J Card Imaging 1989; 3: 57-59.
- 10. Bonte FJ, Christensen EE, Curry CTS III. Tc-99m pertechnetate angiocardiography in the diagnosis of superior mediastinal masses and pericardial effusions. Am J Roentgenol 1969; 107: 404-412.
- 11. Better N, Janicek MJ, Annese M, Kaplan WD. Mediastinal tumor presenting as a cardiac halo on equilibrium radionuclide angiography: a differential diagnosis to pericardial effusion. Clin Nucl Med 1996; 21: 334-335.
- 12. Wang ZJ, Reddy GP, Gotway MB, et al. CT and MR imaging of pericardial disease. Radiographics 2003; 23: S167-S180.
- 13. Shih W-J, Ryo UY, Stipp V, et al. Extracardiac and intrathoracic abnormalities demonstrated by Tc-99m RBC gated blood pool imaging. Radiat Med 1989; 7: 253-256.
- 14. Luis IA, Jimenez-Hoyuela JM, Sloane MH. Pericardial effusion on tomographic MUGA images before adriamycin. J Nucl Cardiol 1997; 4: 569-570.





We thank Dr A Ng for the echo images.
None identified.