Health technology assessment (HTA), as a science, developed in response to concerns about the tide of new technologies overwhelming resource-limited health services. HTA is a multidisciplinary specialty whose purpose is to bring together the evidence to help policymakers, clinicians and patients understand the relative value of technologies.1 It therefore evaluates the effectiveness, the costs, and sometimes the wider impact of health technologies.
There is a thriving international scientific society2 and a global network of more than 40 public-sector agencies3 involved in assessing health technologies (defined as any intervention, including medicines, devices, techniques, and skills, used in the care of patients). The National Institute for Health Research (the research arm of the English National Health Service [NHS]) has a large HTA Programme, established in 1993, which is part of that network. In this article, I outline its structures and functions, and its relations with the National Institute for Health and Clinical Excellence (NICE) and other NHS policymakers.
The HTA Programme was created not to contain new technologies, but because of a commitment by the NHS to evidence-based practice, and to meet the need of purchasing organisations to be informed about appropriateness of new or old technologies.4 Its remit and methods of working are outlined in Box 1. Its aim is to produce robust scientific assessments of the benefits or disbenefits of technologies, new or old, which might be applied in the NHS. HTA focuses on effectiveness in the real world, and is therefore distinct from regulatory approval, which focuses mostly on safety and efficacy, usually in the context of randomised controlled trials.
HTA, as a discipline, often concentrates on new, expensive technologies and on pharmaceuticals,5 but these may not be the priorities of the NHS or of patients. Assessing established technologies, even in established indications (eg, antidepressants for mild depression), can be equally important.6 The HTA Programme therefore describes itself as “needs-led”, and identifies the issues of most importance to the NHS and patients that require further research. Possible topics are identified by widespread consultation, by examining recommendations for further research from Cochrane and other reviews, and with the help of the National Horizon Scanning Centre (which provides government and NHS agencies with advance warnings about important new developments in drugs and devices).7 Over 1000 potential topics per year are prioritised by committees of the NHS and consumer experts, based on criteria of the timeliness of evaluation, the importance of the topic (disease burden, likely effectiveness, cost-effectiveness and total overall cost to the NHS) and the benefits of reducing uncertainty, thereby encouraging either uptake or exclusion of the technology from NHS use.
The HTA Programme then commissions research into the key topics by open tender, usually taken up by academic groups. The topics covered range from stents for abdominal aortic aneurysms8 to mattresses designed to decrease pressure sores,9 as well as many new or old drugs. The Programme commissions around 50 new projects per year, and publishes the outcomes of most (over 380 to date) in its own peer-reviewed journal, Health Technology Assessment. The Programme website (http://www.hta.ac.uk) receives over 16 000 “hits” per week (25% from within the United Kingdom, and 2% from Australia).
Further recent developments have been the addition of a new funding stream reactive to projects suggested by researchers (although the Programme always remains needs-led — a key assessment of such projects will be their prioritisation by the independent NHS committees), and the creation of a new “disease prevention” expert panel to identify technologies in public health (eg, approaches to smoking cessation) for evaluation. The comparators in all projects funded are either established best therapy or current NHS practice, and the key outcomes are patient-focused measures of effectiveness and cost-effectiveness. For instance, in a study of maggots for debriding chronic leg ulcers,10 the comparator was standard modern wound dressings, and the outcomes were rates of healing, cost-effectiveness of healing (measured as cost per ulcer healed and per quality-adjusted life year), and acceptability to patients and to staff.
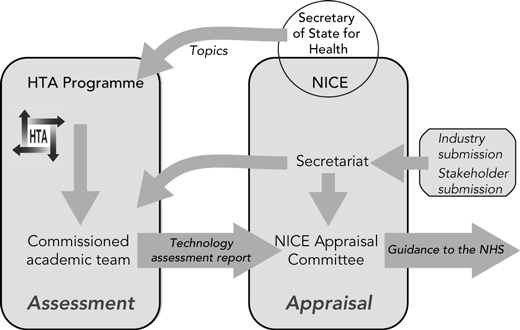
The HTA Programme is scientifically independent of, but accountable to, the Department of Health through the newly established National Institute for Health Research.11 It does not make recommendations as to whether a technology should be adopted or prohibited within the NHS, but aims to inform local policymakers like health care commissioners or national policymakers like the National Screening Committee. A distinction was therefore drawn early on between scientific assessment (the role of the HTA Programme) and judgemental appraisal (the responsibility of policymakers). For instance, NICE is often thought of as an HTA agency, but it is strictly more an appraisal agency in its guidance, reviewing of evidence (usually from the HTA Programme) and consideration of patient and professional views, and application of a judgement. In this way, the scientific assessment is protected as much as possible from the pressures of manufacturing industry, patient groups, politicians, professional bodies, service providers, and clinicians. This is a distinction which is not common among HTA agencies, many of which are government bodies, and which blur the scientific assessment and judgemental appraisal roles.
NICE is an important customer for the HTA Programme (Box 2).12 NICE was established in 1999 to appraise technologies at a national level, thereby avoiding the local variations in approval and practice that are considered unacceptable in a national health service. The topics for NICE are decided by the Secretary of State for Health, based on advice from expert review panels and filtered through a policy review board. The process is managed by NICE itself. The criteria used to select topics are similar to those used in the HTA Programme.
The HTA Programme supports all NICE technology appraisals by commissioning independent assessments of the evidence, accompanied by economic evaluation and a review of manufacturers’ submissions. These are provided to NICE’s appraisal committees to inform its decisions, and are made publicly available once NICE has reached its preliminary decisions. The assessment does not make a recommendation to the committee. In general, NICE’s appraisals are in line with the conclusions of the assessment, although there are cases where evidence presented to the committee by other stakeholders has persuaded them to make decisions contrary to those that the assessment might have anticipated. For instance, an assessment of the drug riluzole, used to treat motor neurone disease, suggested a much higher incremental cost-effectiveness ratio than NICE usually approved, but the appraisal committee accepted arguments from a patient group that there were no other treatments available.13
NICE considers about 15–20 health technologies each year, about two-thirds of which are drugs, although the number is expected to increase with a new streamlined “single technology appraisal” process, also supported by the HTA Programme. The single technology appraisal process alters the burden of proof from the independent HTA report, described above, to the manufacturer, whose submission is carefully critiqued by the independent team commissioned through the HTA Programme. Once approved by NICE, local health authorities are required to provide the technology within 3 months (although there are many difficulties in implementing this14).
NICE also feeds back into the HTA Programme by identifying key areas for further primary research (ie, areas where evidence gaps may affect the outcomes of future revisions of its appraisals), which the HTA Programme may then take up as priorities. An example is photodynamic therapy with verteporfin for macular degeneration, approved by NICE’s appraisal committee despite the lack of evidence of long-term benefit.15 A further randomised controlled trial was considered impractical and possibly unethical, and the HTA Programme is now funding a cohort study to follow up almost all patients having this treatment within the NHS.
The success of the HTA Programme so far is acknowledged in lavish praise from a recent independent but Treasury-sponsored review of health research in the UK.16 The review recommended that the Programme’s work be expanded to take on more clinical trials, and that the possibility of using registries based on the developing electronic patient record system in the UK be considered to support more real-world observational studies of the effectiveness of new technologies. The particular strengths of the English HTA Programme are its being embedded within the NHS while maintaining independence, flexibility and responsiveness to changing demands; the involvement of health care professionals and consumers at every level; and the quality of the work performed. Its weaknesses are the difficulty it has in spotting the problematic technology (which is especially important if expensive and slow primary research has to be commissioned), and the inevitable trade-off between the need for rigour and generalisability on the one hand and context-specificity and immediacy on the other. This means that, like other HTA programs, the English Programme faces increasing challenges in delivering timely analyses that are scientifically robust yet relevant to policy.
Despite this, the overall situation for HTA in England is perhaps less fragmented and has less duplication than in Australia.17 In Australia, there are divisions between federal and state activities, and between areas such as pharmaceuticals (Pharmaceutical Benefits Advisory Committee and others), devices (Prostheses and Devices Committee) and procedures (Medical Services Advisory Committee). There is also confusion over who is responsible for combined technologies such as drug-eluting coronary artery stents, which had a single assessment and appraisal in England compared with a more drawn-out process in Australia. These differences arise largely from the structures of the respective health services, with divisions between who funds what, and the existence of silo budgets in Australia. Although similar problems plagued the NHS for many years and can still occur, in general, the UK NHS is getting better at acting as a corporate whole, drawing on the same (limited) funds. It may be that the establishment of the Health Policy Advisory Committee on Technology (HealthPACT) in Australia would also resolve many of these problems. The separation of assessment and appraisal, and the independence of those conducting the review from policymakers or funders also differ from Australian practice. The limited transparency of Australian HTA and its lack of public involvement have been criticised,16 but are both well addressed in the English system.
1 Remit and working methods of the English National Institute for Health Research Health Technology Assessment Programme
Identifying challenging technologies, and prioritising these for research.
Commissioning high-quality research to answer relevant questions about health technologies.
Monitoring research to ensure that it meets the needs of policymakers, clinicians or patients.
Disseminating the results to relevant audiences.
- Tom Walley1,2
- 1 Department of Pharmacology and Therapeutics, Liverpool University, Liverpool, UK.
- 2 Royal Liverpool University Hospital, Liverpool, UK.
Tom Walley is Director of the National Institute for Health Research Health Technology Assessment Programme.
- 1. Gabbay J, Walley T. Introducing new health interventions. BMJ 2006; 332: 64-65.
- 2. Health Technology Assessment International [website]. http://www.htai.org (accessed Jul 2007).
- 3. INAHTA (International Network of Agencies for Health Technology Assessment) [website]. http://www.inahta.org (accessed Jul 2007).
- 4. Stevens A, Milne R. Health technology assessment in England and Wales. Int J Technol Assess Health Care 2004; 20: 11-24.
- 5. Oliver A, Mossialos E, Robinson R. Health technology assessment and its influence on health-care priority setting. Int J Technol Assess Health Care 2004; 20: 1-10.
- 6. Coulter A. Perspectives on health technology assessment: response from the patient’s perspective. Int J Technol Assess Health Care 2004; 20: 92-96.
- 7. Department of Public Health and Epidemiology. National Health Service. National Institute for Health Research. National horizon scanning centre. http://www.pcpoh.bham.ac.uk/publichealth/horizon/ (accessed Jul 2007).
- 8. EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005; 365: 2179-2186.
- 9. Nixon J, Cranny G, Iglesias C, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006; 332: 1413-1416.
- 10. Raynor P, Dumville J, Cullum N. A new clinical trial of the effect of larval therapy. J Tissue Viability 2004; 14: 104-105.
- 11. Best research for best health: a new national health research strategy. London: Department of Health, 2006.
- 12. Pearson SD, Rawlins MD. Quality, innovation, and value for money: NICE and the British National Health Service. JAMA 2005; 294: 2618-2622.
- 13. Barnett D. Riluzole for motor neurone disease. Reply from chairman of appraisal committee at NICE. BMJ 2001; 323: 573.
- 14. Sheldon TA, Cullum N, Dawson D, et al. What’s the evidence that NICE guidance has been implemented? Results from a national evaluation using time series analysis, audit of patients’ notes, and interviews. BMJ 2004; 329: 999-1004.
- 15. National Institute for Clinical Excellence. TA68 macular degeneration (age related) — photodynamic therapy: guidance. London: NICE, 2003. http://www.nice.org.uk/page.aspx?o=TA068guidance (accessed Jul 2007).
- 16. Cooksey D. A review of UK health research funding. London: Her Majesty’s Treasury, Dec 2006. http://www.hm-treasury.gov.uk./media/4/A/pbr06_cooksey_final_report_636.pdf (accessed Jul 2007).
- 17. Australian Government Productivity Commission. Impacts of advances in medical technology in Australia. Productivity Commission Research Report. 31 August 2005. Melbourne: Productivity Commission, 2005. http://www.pc.gov.au/study/medicaltechnology/finalreport/medicaltechnology.pdf (accessed Jul 2007).





Abstract
The Health Technology Assessment (HTA) Programme in England is a government-funded but independent research program.
It is “needs-led”, identifying technologies of most importance to the National Health Service and commissioning research to provide answers on these technologies useful to policymakers, clinicians and patients.
It is “science-added”, refining problems to researchable questions and working with researchers to ensure that the question is addressed, and disseminating the findings to key audiences.
There is a clear distinction in England between assessment (a scientific process and the role of the HTA Programme) and appraisal (the role of policymakers, like the National Institute for Health and Clinical Excellence).
There are many features common to HTA in Australia and England, but also differences, as HTA in each country has to adapt to its own environment.