Measles was once a common childhood disease in Australia, and medical practitioners were well acquainted with the “fever, generalised maculopapular rash, cough and conjunctivitis” syndrome that equated to a measles diagnosis. Measles complications, particularly bronchopneumonia and otitis media in children, were commonplace. With so many cases in the community, relatively uncommon severe complications, including acute encephalitis (1 in 2000 cases), subacute sclerosing panencephalitis (1 in 25 000 cases), and death, were also encountered.1
The measles virus is a genetically stable, single-stranded RNA virus of the Paramyxoviridae family and Morbillivirus genus, which only naturally infects humans. The virus is highly infectious, with a basic reproductive rate (ie, the average number of secondary infections that would result from one person with measles in a fully susceptible population) of between 10 and 20.2 The only means of preventing infection with the measles virus is vaccination of susceptible individuals.
The introduction in 1968 of an effective live, attenuated vaccine against measles in Australia, and its inclusion in the national childhood vaccination schedule in 1975 (recommended for children aged 15 months in 1971 and scheduled for children aged 12 months in 1975), saw measles become less common, although outbreaks were still regularly experienced. During the 1980s and 1990s, increased attention was given to measles control in Australia. The inclusion of a second vaccine dose, and improving vaccine coverage among preschool-aged children (currently, 94% measles–mumps–rubella [MMR] coverage at 12 months), has resulted in the elimination of endemic measles in Australia.3 Elimination in a particular country or region is achieved when immunity is continually uniformly maintained across the population at a high enough level to ensure that sustained endemic measles transmission cannot occur.
There is, however, no room for complacency. Measles is still endemic in many parts of the world and, despite concerted global control efforts in recent years, tragically remains the fifth leading cause of death among children under 5 years of age worldwide.4 The highly infectious measles virus, which is transmitted by the respiratory route, has an enormous capacity to infect non-immune individuals in the community. Immunisation levels in the order of 95% are necessary to maintain the level of herd immunity required to prevent outbreaks.2 Recent experience demonstrated Australia’s vulnerability to measles importation from countries where measles has not been eliminated. An Australian tour by the Amma (Sri Mata Amritanandamayi Devi) group, based in Kerala, India, over Easter 2006, that included Perth, Melbourne, Sydney and Queensland in its itinerary, resulted in a measles outbreak that affected a number of Australian states, with at least 20 cases in New South Wales alone.5
If the absence of measles in Australia is to be maintained, high coverage with the two-dose schedule must be sustained, and new ways should be explored of raising vaccination coverage in young adults, particularly those living in communal accommodation or travelling internationally. Young adults have often been affected in recent outbreaks and, despite considerable investment, vaccination coverage remains disturbingly low for this age group.3,6 As important is vigilance among the medical community for patients presenting with fever and rash, with the need for rapid appropriate confirmatory testing and prompt public health action to limit spread.

As measles is so infectious, most Australians born before an effective vaccine was introduced have been exposed and are thus considered immune, unless lack of immunity is serologically proven. The introduction of the second dose of MMR vaccine for 10–16-year-olds in 1993 was followed by marked reductions in measles notifications and hospitalisations.3 Further reductions were achieved after the national Measles Control Campaign targeting primary schoolchildren, and the lowering of the age for the second dose of MMR vaccine to preschool-aged children in 1998.3
Even with an effective vaccine (measles vaccine effectiveness is estimated to be 90%–95%), vaccine and vaccination failure can result from: improper storage or transport of the vaccine; incorrect administration into the buttock; administration too early in the infant’s life (when maternal antibodies interfere with the immune response); only giving a single dose; and waning immunity.7,8 As susceptible individuals accumulate in a community, they provide the fuel for an outbreak when infectious measles cases arrive from overseas. In Australia, susceptible individuals now mostly comprise young adults (some of whom are health care workers) and unimmunised children.9
After exposure to an infectious measles case, there is an incubation period of about 10 days (usually between 7 and 18 days) until the appearance of prodromal symptoms, or about 14 days before a rash develops in a susceptible individual. During the prodromal period of 2–4 days, the patient is febrile and miserable, complaining of malaise and loss of appetite. Historically, much has been made of the Koplik spots that may be visible on the buccal mucosa in the molar area, but these are only visible in 60% of cases.10 An infected patient is considered to be infectious from a day before the beginning of the prodrome to 4 days after the onset of rash.
As measles is now rare, most sporadic cases of fever and non-vesiculating rash will not be due to measles; thus, the “measles clinical syndrome” alone has a low positive predictive value and requires laboratory confirmation to guide public health action.11 A host of other infectious agents can present as a febrile illness with a maculopapular rash (see Box 1).12 Useful features for assisting in distinguishing between the various viral exanthems have been reviewed and include characteristic morphology, seasonal occurrence, history of disease contacts, immunisation record, previous exanthematous illnesses, absence of itch, and associated prodromal features.13
Cough and presence of fever at the time of rash onset are important features that increase the specificity of a diagnosis of measles in a patient with rash and fever.14 A recent study in Victoria found that confirmed sporadic measles cases were more likely to report fever at rash onset, cough, conjunctivitis, and year of birth between 1968 and 1981.15
Suspected measles cases must be confirmed, as the public health measures that follow have considerable resource implications. When measures are not promptly initiated, secondary cases will result in unnecessary and potentially serious disease and large outbreaks that consume considerable public health resources. In all patients who present with a fever and non-vesiculating rash, measles must be considered and formally confirmed or excluded as a matter of urgency by specific testing, unless a clinician has confirmed an alternative diagnosis. The attending clinician should contact local public health authorities to discuss case details and the most appropriate diagnostic tests. Testing is important to confirm a case of measles, but equally important to exclude measles, so that patients and their family can be reassured, and unnecessary resource-intensive and disruptive public health action avoided.16 Any malnourished child who is diagnosed with measles should be provided with vitamin A supplements to decrease the likelihood and severity of complications.
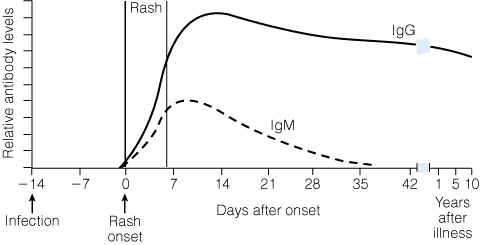
The accepted standard for confirming measles is by detecting measles-specific IgM antibody in venous blood, which appears within the first few days after rash onset, rapidly increases and then declines, becoming undetectable after 4–12 weeks (Box 2).17 Measles IgM is detectable in serum by standard assays on Day 3 after rash onset in about 70% of measles cases and by Day 7 in almost all cases. Measles IgG antibody testing should be requested on the same specimen to rule out previous infection or immunisation. If an assay for measles IgM is negative within 3 days of rash onset, then repeat samples should be taken. A measles vaccination history should always be ascertained. In a previously vaccinated patient, in whom measles is considered the likely clinical diagnosis, the need for further serological testing should be discussed with the pathologist. Testing for other pathogens, such as rubella virus and parvovirus B19, should also be considered.18
A positive result of a measles-specific IgM antibody assay may indicate measles infection, recent vaccination or be a false positive result.19 In Australia, where immunisation coverage is high and disease is rare, a positive IgM antibody result has a reduced positive predictive value. The measurement of measles IgG titres on paired samples can help rule out a false positive IgM result. If IgG determination occurs within 7 days of rash onset and then again 3–4 weeks after rash onset, seroconversion or a fourfold increase in measles IgG titre confirms measles infection, although the need for public health action will have been decided before this. If doubt remains about a positive measles IgM result, a measles enzyme immunoassay with a different format to the primary test, or a reverse transcriptase-polymerase chain reaction (RT-PCR), can be used as confirmatory assays.
At least 5% of primary measles vaccinations can result in a febrile rash illness, with a positive result for measles IgM antibody.20 The illness is most likely to be vaccine-related if there is no cough, the rash began 7–14 days after MMR vaccination, and no source or secondary cases have been identified.
Measles virus RNA can be detected in nasopharyngeal aspirates or nose and throat swabs using RT-PCR for up to 3 weeks after rash onset, or by immunofluorescence or viral culture for 1–2 days in the same specimens. However, RT-PCR, which is highly specific, is not currently routinely available for primary diagnosis in Australia;21 culture is no longer routinely used for measles diagnosis in Australia; and immunofluorescence is not an ideal test, as false positive and false negative results are common. Thus, although immunofluorescence may prove a useful additional test in the first few days after rash onset, when measles IgM may be undetectable, it is preferable to repeat measles IgM and IgG antibody testing of peripheral blood after a week, if these tests were initially negative.
To confirm elimination of endemic measles virus, it is necessary to genotype confirmed measles diagnoses in Australia. From all IgM-confirmed measles cases, early-catch urine, a nasopharyngeal aspirate, combined nose and throat swab, or heparinised blood (collected within 4 days of rash onset, and preferably transported at 4°C but not frozen) should be sent to a reference laboratory for RT-PCR and sequencing. This allows the vaccine virus to be distinguished from wild-type virus, and also allows the geographical origin of measles importations to be traced.22
People who have had close contact with confirmed measles cases need to be assessed as to their risk of measles by public health authorities. Anyone born in Australia before 1966 is considered immune unless they have serological evidence to indicate otherwise.23 Similarly, any immunocompetent individual who has received two doses of MMR vaccine at least 3 weeks before exposure is considered immune, as is anyone with a history of definite measles infection documented by a health care provider.
MMR within the first 3 days is effective, as the incubation period of the vaccine strain (4–6 days) is shorter than the incubation period of wild-type measles virus (10–14 days). Although MMR is generally only offered to children older than 9 months, this age limit may occasionally be reduced in outbreak situations. As maternal antibodies may result in an inadequate response below 1 year of age, two subsequent MMR doses should be administered according to the National Health and Medical Research Council vaccination schedule.23
NHIG should be considered if it can be administered between 3 and 7 days of exposure. High-risk contacts who are most likely to benefit are individuals who are severely immunocompromised, pregnant women with negative results for measles IgG, and children between 6 and 9 months of age. For infants less than 6 months of age, NHIG administration should be considered if their mother has been diagnosed with measles or is seronegative.24 It is recommended that MMR should not be administered until at least 3 months after administration of NHIG, because of potential interference with the live vaccine.
Individuals with measles are generally too unwell to undertake normal activities. Adults should be strongly encouraged to isolate themselves at home until 4 days after rash onset. Children with measles must be excluded from school or child care for this period.25 Immunised and other immune contacts do not need to be excluded. Public health legislation in some states requires that unimmunised contacts of a case be excluded from child care or school until 14 days after the first day of rash in the last case in an outbreak, unless they were vaccinated within 72 hours of first contact with the infectious case. The attending specialist should determine the management of immunocompromised contacts on a case-by-case basis.
- David N Durrheim1
- Heath Kelly2,3
- Mark J Ferson4,5
- David Featherstone6
- 1 Hunter New England Population Health, Hunter New England Area Health Service, Newcastle, NSW.
- 2 Epidemiology Unit, Victorian Infectious Diseases Reference Laboratory, Melbourne, VIC.
- 3 School of Population Health, University of Melbourne, Melbourne, VIC.
- 4 Public Health Unit, South Eastern Sydney and Illawarra Area Health Service, Wollongong, NSW.
- 5 School of Public Health and Community Medicine, University of New South Wales, Sydney, NSW.
- 6 Vaccine-Preventable Disease Laboratory Network, Expanded Programme for Immunization, World Health Organization, Geneva, Switzerland.
We thank Dr Michaela Riddell, National Health and Medical Research Council Sidney Sax Fellow, and Senior Laboratory Epidemiologist, Victorian Infectious Diseases Reference Laboratory, for constructive comments on a draft of this manuscript.
None identified.
- 1. Tobin S, Kelly H. Measles encephalitis in Victoria: down but not out. Aust N Z J Public Health 1999; 23: 443.
- 2. Anderson RM, May RM. Infectious diseases of humans. Dynamics and control. Oxford: Oxford University Press, 1992: 70.
- 3. Gidding HF, Wood J, MacIntyre CR, et al. Sustained measles elimination in Australia and priorities for long term maintenance. Vaccine 2007; 25: 3574-3580.
- 4. Strebel P, Cochi S, Grabowsky M, et al. The unfinished measles immunization agenda. J Infect Dis 2003; 187 Suppl 1: S1–S7.
- 5. Communicable Diseases Report for New South Wales for March and April 2006. N S W Public Health Bull 2006; 17: 88–94.
- 6. Gidding HF. The impact of Australia’s measles control programme over the past decade. Epidemiol Infect 2005; 133: 99-105.
- 7. Wood DL, Brunell PA. Measles control in the United States: problems of the past and challenges for the future. Clin Microbiol Rev 1995; 8: 260-267.
- 8. Kremer JR, Schneider F, Muller CP. Waning antibodies in measles and rubella vaccines — a longitudinal study. Vaccine 2006; 24: 2594-2601.
- 9. Riddell MA, Lynch P, Jin L, Chibo D. Measles case imported from Europe to Victoria, Australia, March 2006. Euro Surveill 2006; 11: E060518.2.
- 10. Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189 Suppl 1: S4-S16.
- 11. Lambert SB, Kelly HA, Andrews RM, et al. Enhanced measles surveillance during an interepidemic period in Victoria. Med J Aust 2000; 172: 114-118. <MJA full text>
- 12. Weber DJ, Cohen MS, Fine J-D. The acutely ill patient with fever and rash. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious disease. 5th ed. Philadelphia: Churchill Livingstone, 2000: 633-650.
- 13. Scott LA, Stone MS. Viral exanthems. Dermatol Online J 2003; 9: 4.
- 14. Ferson MJ, Young LC, Robertson PW, Whybin LR. Difficulties in the clinical diagnosis of measles: proposal for modified clinical case definition. Med J Aust 1995; 163: 364-366.
- 15. Wang YJ, Andrews RM, Lambert SB. Measles surveillance in Victoria, Australia. Bull World Health Organ 2006; 84: 105-111.
- 16. Dayan GH, Ortega-Sanchez IR, LeBaron CW, Quinlisk MP; Iowa Measles Response Team. The cost of containing one case of measles: the economic impact on the public health infrastructure — Iowa, 2004. Pediatrics 2005: 116: e1-e4.
- 17. Monitoring the interruption of indigenous measles transmission, Cape Town meeting, 14 October 2003. Wkly Epidemiol Rec 2004; 79: 70-72.
- 18. Kelly H, Leydon J. Outbreaks caused by parvovirus B19. Euro Surveill 2005; 10: E1-E2.
- 19. Dietz V, Rota J, Izurieta H, et al. The laboratory confirmation of suspected measles cases in settings of low measles transmission: conclusions from the experience in the Americas. Bull World Health Organ 2004; 82: 852-857.
- 20. Jenkin GA, Chibo D, Kelly H, et al. What is the cause of rash in a child following measles–mumps–rubella vaccination? Med J Aust 1999; 171: 194-197.
- 21. Riddell M, Chibo D, Kelly HA, et al. Investigation of optimal specimen type and sampling time for the detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39: 375-376.
- 22. Featherstone D, Brown D, Sanders R. Development of the Global Measles Laboratory Network. J Infect Dis 2003; 187 Suppl 1: S264-S269.
- 23. National Health and Medical Research Council. The Australian immunisation handbook. 8th ed. Canberra: Australian Government Department of Health and Ageing, 2003: 155. http://www9.health.gov.au/immhandbook/ (accessed Jun 2007).
- 24. Ferson MJ, Young LC, Robertson PW, Whybin LR. Difficulties in clinical diagnosis of measles: proposal for modified clinical case definition. Med J Aust 1995; 163: 364-366.
- 25. National Health and Medical Research Council. Staying healthy in child care. Preventing infectious diseases in child care. 4th ed. Canberra: NHMRC, 2005: 8. http://www.nhmrc.gov.au/publications/synopses/_files/ch43.pdf (accessed Jun 2007).





Abstract
Measles is now rare in Australia, and cases can usually be linked to its importation from endemic countries.
To prevent measles outbreaks in Australia, high vaccination coverage with two doses of vaccine must be sustained.
All medical practitioners should consider a diagnosis of measles in a patient of any age who presents with fever and a non-vesiculating, non-itchy rash.
If measles is suspected clinically, public health authorities should be immediately notified, so that testing and management of patients can be discussed and contact tracing initiated.
When a patient is suspected of having measles, testing of a serum sample for measles-specific IgM and IgG antibodies should be requested urgently.
Pathology laboratories should have effective protocols for immediately reporting positive measles-specific IgM antibody tests, or other results indicative of measles, to public health authorities.