Sodium valproate (SVP) is widely used for treating epilepsy, pain syndromes and psychiatric disturbance. Following its introduction in 1960,1 many studies found that there was a poor correlation between serum SVP concentration and dose. As a result, measurement of serum SVP concentration has been in routine clinical use for over 25 years, with a suggested therapeutic range of 300–700 μmol/L.2
Measurement of a trough level is required, so the blood sample has to be taken at least 8 hours after the most recent dose. This requirement is often not met in busy clinical settings and, even when it is, studies have still found a poor correlation between SVP dose and serum concentration.3-7
Several studies have further suggested that serum SVP concentration bears little relation to clinical effectiveness (eg, seizure control) or to the magnitude of side effects.8,9 In fact, a patient’s dose of SVP can be adjusted on the basis of clinical parameters (ie, effectiveness in controlling symptoms set against magnitude of side effects). Thus, for day-to-day use, measuring serum SVP concentration is unnecessary.2,4,10-13 Current evidence suggests that measuring SVP is not clinically useful except in certain situations such as the assessment of toxicity or compliance7,14,15 or, possibly, in the alteration of dosage for individual patients on polypharmacy.2 In spite of this, the practice of routine measurement of serum SVP concentration is widespread, particularly in emergency departments (EDs), outpatient departments and general practice settings, and many clinicians are unaware of the significance of measuring trough levels. Audits elsewhere16-20 have repeatedly indicated inappropriate use of the test.
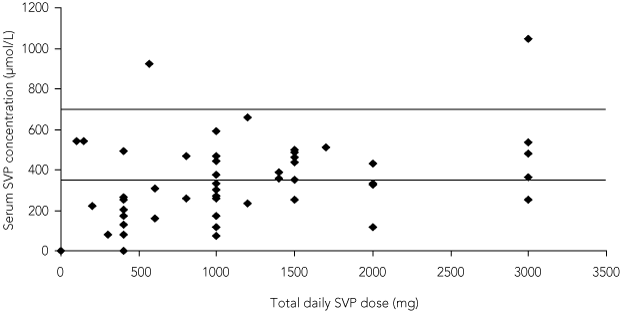
Twenty-six of 89 samples (29%) were valid. The results of all samples are plotted against dose in Box 3. The poor correlation between dose and serum level was unaffected by whether or not the test was performed at the correct time.
Five of the 89 sets of notes could not be satisfactorily assessed in terms of the effect of SVP testing on patient management. Among the remaining 84, 48 results were clearly documented in the patient notes, with 18 management changes noted. However, 36 tests (43% of tests recorded in the 84 sets of notes analysed) apparently made no difference to patient management. Hence, at most, 57% of the tests reviewed had an effect on clinical management (Box 2).
- Chamishani Rathmalgoda1
- Julia M Potter2,3
- Christian J Lueck2,3
- 1 University of New South Wales, Sydney, NSW.
- 2 The Canberra Hospital, Canberra, ACT.
- 3 Australian National University Medical School, Canberra, ACT.
We wish to thank The Canberra Hospital Private Practice Fund for supporting our study through a Summer Vacation Scholarship.
Christian Lueck has received speaker fees for lecturing on various aspects of stroke to general practitioners and travel assistance to attend Stroke Society of Australasia meetings from Sanofi-Aventis, which manufactures sodium valproate. Sanofi-Aventis also provided an unrestricted grant to The Canberra Hospital Stroke Unit when it was formed in 2004; provides annual support for the Australian and New Zealand Association of Neurologists’ training weekend for advanced trainees in neurology (a course organised by C L); and supports an annual training weekend in neuro-ophthamology (also organised by C L).
- 1. Shorvon S. Handbook of epilepsy treatment. 2nd ed. Oxford: Blackwell, 2005.
- 2. Arroyo S. Valproate. In: Shorvon S, Perucca E, Fish DR, Dodson E, editors. The treatment of epilepsy. 2nd ed. Oxford: Blackwell, 2004.
- 3. Lenn NJ, Robertson M. Clinical utility of unbound antiepileptic drug blood levels in the management of epilepsy. Neurology 1992; 42: 988-990.
- 4. Anthony M, Hinterberger H, Lance JW. Plasma sodium valproate levels and clinical response in epilepsy. Clin Exp Neurol 1977; 14: 208-215.
- 5. Vajda FJ, Mihaly GW, Miles JL, et al. Sodium valproate: dose–plasma level relationships and interdose fluctuations. Clin Exp Neurol 1978; 15: 145-153.
- 6. Rowan AJ, Binnie CD, de Beer-Pawlikowski NK, et al. Sodium valproate: serial monitoring of EEG and serum levels. Neurology 1979; 29: 1450-1459.
- 7. Mehrotra TN, Aneja GK, Arora V, et al. Valproate sodium in epilepsy. A clinical trial including monitoring of drug levels. J Assoc Physicians India 1990; 38: 277-279.
- 8. Gram L, Flachs H, Würtz-Jørgensen A, et al. Sodium valproate, serum level and clinical effect in epilepsy: a controlled study. Epilepsia 1979; 20: 303-311.
- 9. Ishikawa T, Ogino C, Furuyama M, et al. Serum valproate concentrations in epileptic children with favourable responses. Brain Dev 1987; 9: 283-287.
- 10. Lundberg B, Nergardh A, Boreus LO. Plasma concentrations of valproate during maintenance therapy in epileptic children. J Neurol 1982; 228: 133-141.
- 11. Jannuzzi G, Cian P, Fattore C, et al. A multicentre randomised controlled trial on the clinical impact of therapeutic drug monitoring in patients with newly diagnosed epilepsy. The Italian TDM Study Group in Epilepsy. Epilepsia 2000; 41: 222-230.
- 12. Scottish Intercollegiate Guideline Network. Diagnosis and management of epilepsy in adults. A national clinical guideline. Guideline No. 70. Edinburgh: SIGN, 2003. http://www.sign.ac.uk/pdf/sign70.pdf (accessed Aug 2007).
- 13. Eadie MJ. Indications for plasma drug monitoring in patients with epilepsy. Implications for reducing costs. Pharmacoeconomics 1997; 11: 343-349.
- 14. Bowden CL, Janicak PG, Orsulak P, et al. Relation of serum valproate concentration to response in mania. Am J Psychiatry 1996; 153: 765-770.
- 15. Larkin JG, Herrick AL, McGuire GM, et al. Antiepileptic drug monitoring at the epilepsy clinic: a prospective evaluation. Epilepsia 1991; 32: 89-95.
- 16. Schoenenberger RA, Tanasijevic MJ, Jha A, Bates DW. Appropriateness of antiepileptic drug level monitoring. JAMA 1995; 274: 1622-1626.
- 17. Sharpe PC, Morrow J, Trimble ER. An audit of therapeutic drug monitoring of anticonvulsants. Ulster Med J 1995; 64: 151-156.
- 18. Thapar A, Richens A, Roland M, et al. Are serum anticonvulsant levels in people with epilepsy appropriately monitored? J Eval Clin Pract 2001; 7: 335-338.
- 19. Affolter N, Krahenbul S, Schlienger RG. Appropriateness of serum level determinations of antiepileptic drugs. Swiss Med Wkly 2003; 133: 591-597.
- 20. Walters RJ, Hutchings AD, Smith DF, Smith PE. Inappropriate requests for serum anti-epileptic drug levels in hospital practice. QJM 2004; 97: 337-341.





Abstract
Objective: To assess whether serum sodium valproate (SVP) testing in the hospital setting is being performed according to evidence-based criteria.
Design and setting: Retrospective audit of serum SVP concentration measurements performed on inpatients and emergency department patients at The Canberra Hospital from May to July 2005.
Main outcome measures: Indication for performing the test, assessed against evidence-based criteria; timing of blood sample collection; whether the test result altered patient management; whether the request form allowed laboratory staff to assess the appropriateness of the test; cost of performing inappropriate tests.
Results: We retrieved 211 test results performed on a total of 95 patients. Notes on 89 patients were available for analysis. Based on evidence-based criteria, 15% of tests were done for an appropriate indication and 29% of the samples were taken at an appropriate time. At most (using generous criteria), 57% of test results made a difference to patient management. Forty-four per cent of request forms contained sufficient detail to allow the pathology department to assess the appropriateness of the test. An estimated $13 236 would be spent unnecessarily on SVP testing at our hospital over a 1-year period.
Conclusions: Most serum SVP level measurements were requested inappropriately, and many were not taken at the correct time, thereby rendering the results uninterpretable. Better education of requesting clinicians could significantly reduce the number of unnecessary tests and thus reduce the cost to the health service.