Treating respiratory tract infections is one of the most common reasons for prescribing antibiotics. However, antibacterial therapy is often unnecessary, particularly for viral or other upper respiratory tract infections, and the inappropriate use of antibiotics is closely linked to the emergence of resistance in key respiratory and other pathogens.1,2 Identifying the aetiology of lower respiratory tract infections (LRTIs) has traditionally been slow. Bacterial cultures take at least a day, viral cultures even longer, and, even with aggressive investigation for aetiology, the cause is found only about half the time.3 Thus, treatment of LRTIs is generally empirical, with clinicians reliant on previous aetiology and microbiology studies to guide their choice of therapy, although each of these can be misleading. In Australia, there has been only one published study of the aetiology of community-acquired pneumonia (CAP) — a 1989 study that assessed 106 patients in a single hospital.4 Thus, for the most part, clinicians have depended on international data, which may have uncertain relevance to Australia. Published resistance rates may be misleading. For example, the rate of resistance in consecutive isolates of Streptococcus pneumoniae must be tempered by the knowledge that this organism can be the cause of anywhere between 8% and 75% of episodes of CAP,5 and there has never been a documented microbiological failure when treating pneumococcal pneumonia or bacteraemia with adequate doses of penicillin.6 Although some retrospective studies suggest that combination therapy (such as a β-lactam and a macrolide) may reduce mortality in pneumococcal infections, this is yet to be confirmed in prospective studies.7
One way to help address these issues is to use new, rapid, point-of-care diagnostic techniques. If a general practitioner or emergency physician is quickly able to identify that a respiratory tract infection is of viral origin, then he or she may feel more confident about not prescribing antibiotics and may be able to institute appropriate isolation precautions or even specific antiviral therapy. Early identification that the cause is pneumococcal can allow the clinician to be comfortable treating with a simple, cheap narrow-spectrum β-lactam such as benzylpenicillin or amoxycillin in conjunction with either a macrolide or doxycycline, in line with the recommendations of the Australian antibiotic guidelines.8 Rapid diagnosis of CAP caused by Legionella can facilitate the early institution of appropriate therapy for this potentially fatal disease.9
A summary of rapid point-of-care tests (POCTs) is given in Box 1.
Benefits of POCTs: Obtaining a rapid diagnosis of influenza has several potential benefits. Less money is spent on other investigations, antibiotic use may be reduced, and length of stay in the emergency department and hospital may be shortened.30 As anti-influenza medications have greater efficacy if started within 48 hours of symptom onset, rapid diagnosis allows the more judicious use of these medications, which is especially useful in view of the current scarcity of some of these agents.31 With the current concern about the threat of pandemic influenza from the H5N1 avian strain affecting many parts of the world, the availability of rapid and accurate tests for influenza is particularly desirable. For patients in hospitals or supported accommodation facilities, early diagnosis allows the appropriate use of infection control methods, which can reduce the number of secondary cases within the institution.30,32
Limitations of POCTs: The sensitivity of POCTs for influenza is lower than that of other tests such as the polymerase chain reaction (PCR) or viral culture. Also, POCTs do not give information on the influenza subtype, and it is not yet clear whether all tests will detect H5N1 viruses. As they do not contribute to knowledge of circulating strains, it is important to confirm diagnoses — particularly in the non-epidemic situation — with other, more accurate tests such as PCR. This requires a second sample being obtained from the patient. POCTs generally have a higher sensitivity in children than adults and for nasopharyngeal aspirates (NPAs), which can be more difficult and unpleasant to obtain, rather than throat swabs.13 In addition, the tests are not currently Medicare rebatable when used at the point of care and have a shelf life of about 12–18 months, so cost is certainly an issue. Overall, they are probably more useful at times when the likelihood of influenza is relatively low, such as between epidemics. In times of higher likelihood of influenza illness, empirical antiviral treatment is likely to be more cost-effective.32
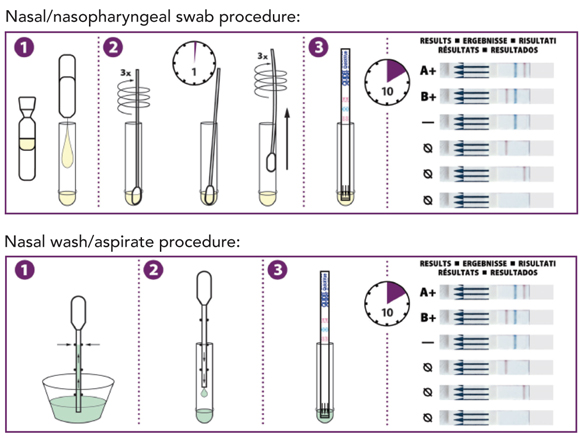
The QuickVue Influenza A + B test (Quidel Corporation, San Diego, Calif, USA) is an enzyme immunoassay that uses monoclonal antibodies against various influenza A and B antigens. The antibodies are impregnated in a test strip. Reagent solution is added to the provided extraction tube, which is then shaken to dissolve the contents. A nasal swab is inserted into the tube and rotated and left for 1 minute before being removed. Alternatively, a dropper is provided that can be used to add fluid from nasal washings or an NPA directly to the extraction tube in place of the nasal swab. The test strip is then inserted into the tube and left for 10 minutes. Influenza virus antigens react with reagents in the test strip, with positive results signified by a pink or red line (Box 2). Sensitivity is 74%–95% early in the illness, but decreases with each day of illness and possibly also if the test is used after antiviral medications are initiated. Specificity is initially 76%–100%, but decreases if the test is delayed.10-12 QuickVue A + B is able to distinguish between influenza A and B viruses.
Sensitivity of the test is 62%–82% for influenza A and 58%–71% for influenza B. Specificities are 92%–100%.13-15 This is the simplest of the rapid tests for the person doing the test and, like the QuickVue test, can distinguish between influenza A and B viruses.
Compared with viral culture, immunofluorescence and PCR, the test has only moderate sensitivity (65%–77%), but specificity is better (77%–98%).11,16 The test requires more hands-on time than the QuickVue A + B test.11 It is not available in Australia and it appears that it will be superseded by a newer version soon.
The ZstatFlu-II test (ZymeTx Inc, Oklahoma City, Okla, USA) is a chemiluminescent rapid diagnostic test, which also detects the enzymatic activity of influenza neuraminidase. When a throat swab containing influenza virus is added to the device, the viral enzyme hydrolyses a synthetic substrate, leading to release of chemiluminescent reporter groups. The specimen is left for 15 minutes and then mixed internally with sodium hydroxide to terminate the reaction. A positive reaction releases light, which is captured on a Polaroid high-speed detector instant film within the imaging device. This shows up as a white “plus” sign on the black background of the film.10,17 The absence of the plus sign (ie, a completely black film) indicates a negative result. The test can not distinguish between influenza A and B, and there is no crossreactivity with the neuraminidase of parainfluenza viruses. One small advantage of the test is that the result can be stored as a permanent record.
Compared with viral culture, the test has a sensitivity of 50%–88% and a specificity of 83%–100%.10,17 The recommended specimen is a throat swab, but sensitivity and specificity of the test are higher when nasal aspirates are used. The test takes about 30 minutes. At the time of writing, ZstatFlu-II was not yet available in Australia.
Other available tests for rapid diagnosis of influenza include the BioStar OIA FLU A/B test (BioStar, Boulder, Colo, USA) and the Directigen EZ Flu A + B test (Becton Dickinson, Franklin Lakes, NJ, USA). However, the OIA test is sufficiently complex to be less likely to be used by clinicians, and the Directigen EZ test has poorer sensitivity results than those given above.33 The Directigen EZ test is not licensed for office use in the United States.
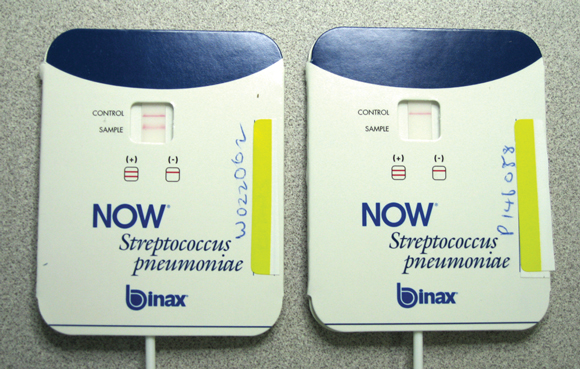
The BinaxNOW Streptococcus pneumoniae urinary antigen test (Binax, Scarborough, Me, USA) is a rapid ICT assay performed on standard urine samples. A swab is dipped in the urine and then placed on a nitrocellulose membrane that contains rabbit antibodies against S. pneumoniae. Six drops of a supplied reagent solution are added, and after 15 minutes the card is read. There is one line for the internal control, and the appearance of a second line signifies a positive result (Box 3). The test detects the C-polysaccharide from the cell wall of all pneumococcal serotypes.
Sensitivity is 70%–92% for patients with bacteraemic pneumococcal pneumonia and is at the higher end of the range for those with more severe infection.18-21 Timing of the test in relation to disease onset is not critical, as the test can remain positive for a month or more after pneumococcal infection.20 Specificity is higher than 90%.18,19,21 The test is useful for diagnosing pneumococcal infections rapidly — particularly in people who have already received antibiotics as, unlike cultures of blood and sputum, antibiotics do not appear to affect the test’s accuracy. The test appears to be less useful in children because nasopharyngeal colonisation with S. pneumoniae can lead to false positive results.22 A more recently identified use of the pneumococcal antigen assay is in the diagnosis of S. pneumoniae meningitis, when it can be used on cerebrospinal fluid rather than urine. In this setting, it can detect pneumococcal meningitis with sensitivity and specificity of over 95%.23
The BinaxNOW Legionella urinary antigen test (Binax, Scarborough, Me, USA) is another ICT assay, performed on a standard urine sample. It is very similar to the pneumococcal urinary antigen test, again taking 15 minutes, with the only difference being that two drops of the reagent are required rather than six. It only detects L. pneumophila serogroup 1, but, as most Legionella infections are caused by this serogroup, it provides rapid diagnosis for most cases of CAP caused by Legionella.24 Sensitivity and specificity are 70%–80% and 97%–100%, respectively.24 As cultures for Legionella take 3–5 days and confirmation of serological conversion takes 4–8 weeks, the rapidity of the BinaxNOW test makes it very useful, particularly in outbreaks of Legionnaires’ disease. Another benefit is that some patients with legionellosis are unable to produce an adequate sputum specimen, whereas urine is readily obtainable. As Legionella infections may be severe, test positivity is a marker of potentially severe CAP. Thus, the test should be performed on all patients being admitted with CAP.9
POCTs for RSV require a nasopharyngeal swab, NPA or nasal washings. These are intended for use in neonatal or paediatric populations and are less accurate when used in adults.25 The benefits of such tests are the rapid institution of isolation precautions to reduce nosocomial transmission, a possible reduction in antibiotic use, and the option of using specific antiviral therapy such as ribavirin.26,29 The simplest test is the BinaxNOW RSV test (Binax, Scarborough, Me, USA), which is performed in the same way as the BinaxNOW influenza test. Other simple tests for RSV are the SAS RSV test (SA Scientific, San Antonio, Tex, USA), the Clearview RSV test (Unipath Limited, Bedford, UK), and the QuickVue RSV test (Quidel Corporation, San Diego, Calif, USA). Like the POCTs for influenza, sensitivities are moderate but generally over 75%, and specificities are over 90%.25-29
PCR tests can be performed on many types of respiratory specimens and have the advantage of being able to test concurrently for many respiratory viruses with accuracy greater than the POCTs.35 However, they are more expensive, are not fully Medicare rebatable, and take longer to provide a result, mainly because of delays in specimen transport.
- Patrick G P Charles1
- M Lindsay Grayson1,2,3
- 1 Department of Infectious Diseases, Austin Health, Melbourne, VIC.
- 2 Department of Medicine, University of Melbourne, Melbourne, VIC.
- 3 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
None identified.
- 1. Wise R, Hart T, Cars O, et al. Antimicrobial resistance. BMJ 1998; 317: 609-610.
- 2. Gould IM. A review of the role of antibiotic policies in the control of antibiotic resistance. J Antimicrob Chemother 1999; 43: 459-465.
- 3. Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001; 163: 1730-1754.
- 4. Lim I, Shaw DR, Stanley DP, et al. A prospective hospital study of the aetiology of community-acquired pneumonia. Med J Aust 1989; 151: 87-91.
- 5. Moellering RC Jr. The continuing challenge of lower respiratory tract infections. Clin Infect Dis 2004; 38 Suppl 4: S319-S321.
- 6. Garau J. Treatment of drug-resistant pneumococcal pneumonia. Lancet Infect Dis 2002; 2: 404-415.
- 7. Waterer GW. Monotherapy versus combination antimicrobial therapy for pneumococcal pneumonia. Curr Opin Infect Dis 2005; 18: 157-163.
- 8. Antibiotic Expert Group. Therapeutic guidelines: antibiotic. 13th ed. Melbourne: Therapeutic Guidelines Limited, 2006.
- 9. Lettinga KD, Verbon A, Weverling GJ, et al. Legionnaires’ disease at a Dutch flower show: prognostic factors and impact of therapy. Emerg Infect Dis 2002; 8: 1448-1454.
- 10. Pachucki CT. Rapid tests for influenza. Curr Infect Dis Rep 2005; 7: 187-192.
- 11. Rodriguez WJ, Schwartz RH, Thorne MM. Evaluation of diagnostic tests for influenza in a pediatric practice. Pediatr Infect Dis J 2002; 21: 193-196.
- 12. Bellei N, Benfica D, Perosa AH, et al. Evaluation of a rapid test (QuickVue) compared with the shell vial assay for detection of influenza virus clearance after antiviral treatment. J Virol Methods 2003; 109: 85-88.
- 13. Storch GA. Rapid diagnostic tests for influenza. Curr Opin Pediatr 2003; 15: 77-84.
- 14. Booth S, Baleriola C, Rawlinson WD. Comparison of two rapid influenza A/B test kits with reference methods showing high specificity and sensitivity for influenza A infection. J Med Virol 2006; 78: 619-622.
- 15. Cruz AT, Cazacu AC, McBride LJ, et al. Performance characteristics of a rapid immunochromatographic assay for detection of influenza virus in children during the 2003 to 2004 influenza season. Ann Emerg Med 2006; 47: 250-254.
- 16. Rawlinson WD, Waliuzzaman ZM, Fennell M, et al. New point of care test is highly specific but less sensitive for influenza virus A and B in children and adults. J Med Virol 2004; 74: 127-131.
- 17. Hamilton MS, Abel DM, Ballam YJ, et al. Clinical evaluation of the ZstatFlu-II test: a chemiluminescent rapid diagnostic test for influenza virus. J Clin Microbiol 2002; 40: 2331-2334.
- 18. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol 2003; 41: 2810-2813.
- 19. Roson B, Fernandez-Sabe N, Carratala J, et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis 2004; 38: 222-226.
- 20. Marcos MA, Jimenez de Anta MT, de la Bellacasa JP, et al. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J 2003; 21: 209-214.
- 21. Gutierrez F, Masia M, Rodriguez JC, et al. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin Infect Dis 2003; 36: 286-292.
- 22. Dowell SF, Garman RL, Liu G, et al. Evaluation of Binax NOW, an assay for the detection of pneumococcal antigen in urine samples, performed among pediatric patients. Clin Infect Dis 2001; 32: 824-825.
- 23. Marcos MA, Martinez E, Almela M, et al. New rapid antigen test for diagnosis of pneumococcal meningitis. Lancet 2001; 357: 1499-1500.
- 24. Stout JE, Yu VL. Legionellosis. N Engl J Med 1997; 337: 682-687.
- 25. Ohm-Smith MJ, Nassos PS, Haller BL. Evaluation of the Binax NOW, BD Directigen, and BD Directigen EZ assays for detection of respiratory syncytial virus. J Clin Microbiol 2004; 42: 2996-2999.
- 26. Zheng X, Quianzon S, Mu Y, Katz BZ. Comparison of two new rapid antigen detection assays for respiratory syncytial virus with another assay and shell vial culture. J Clin Virol 2004; 31: 130-133.
- 27. Jonathan N. Diagnostic utility of BINAX NOW RSV — an evaluation of the diagnostic performance of BINAX NOW RSV in comparison with cell culture and direct immunofluorescence. Ann Clin Microbiol Antimicrob 2006; 5: 13.
- 28. Kuroiwa Y, Nagai K, Okita L, et al. Comparison of an immunochromatography test with multiplex reverse transcription-PCR for rapid diagnosis of respiratory syncytial virus infections. J Clin Microbiol 2004; 42: 4812-4814.
- 29. Slinger R, Milk R, Gaboury I, Diaz-Mitoma F. Evaluation of the QuickLab RSV test, a new rapid lateral-flow immunoassay for detection of respiratory syncytial virus antigen. J Clin Microbiol 2004; 42: 3731-3733.
- 30. Bonner AB, Monroe KW, Talley LI, et al. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 2003; 112: 363-367.
- 31. Ison MG, Hayden FG. Therapeutic options for the management of influenza. Curr Opin Pharmacol 2001; 1: 482-490.
- 32. Church DL, Davies HD, Mitton C, et al. Clinical and economic evaluation of rapid influenza A virus testing in nursing homes in Calgary, Canada. Clin Infect Dis 2002; 34: 790-795.
- 33. Weinberg A, Walker ML. Evaluation of three immunoassay kits for rapid detection of influenza virus A and B. Clin Diagn Lab Immunol 2005; 12: 367-370.
- 34. Benson RF, Tang PW, Fields BS. Evaluation of the Binax and Biotest urinary antigen kits for detection of Legionnaires’ disease due to multiple serogroups and species of Legionella. J Clin Microbiol 2000; 38: 2763-2765.
- 35. Druce J, Tran T, Kelly H, et al. Laboratory diagnosis and surveillance of human respiratory viruses by PCR in Victoria, Australia, 2002–2003. J Med Virol 2005; 75: 122-129.





Abstract
Many lower respiratory tract infections (LRTIs) are caused by organisms that do not require antibiotics or could be safely treated with narrow-spectrum antibiotics.
Reducing the unnecessary use of antibiotics, particularly broad-spectrum agents, could reduce costs and side effects and delay the emergence of antibiotic-resistant organisms.
Various point-of-care tests are becoming available to help clinicians identify the cause of LRTIs at the time of consultation.
Point-of-care tests can be used to diagnose influenza, pneumococcal infections, Legionella and respiratory syncytial virus infections, thus allowing early decisions to be made on appropriate management.