Despite heightened public awareness that obesity is a major risk factor for cardiac disease and death,1 the prevalence of obesity (a body mass index [BMI] > 30 kg/m2) is increasing in Australia and other developed nations.2,3 Obesity has also been considered a major risk factor in patients undergoing cardiac surgery.4,5 Patients who are obese are likely to have other comorbidities,6 including diabetes mellitus, hypertension, hyperlipidaemia, and coronary artery disease, and pose technical difficulties in operative and perioperative care that may contribute to poorer early outcomes. More recently, however, several overseas studies have suggested that there may be little association between obesity and early mortality and morbidity after cardiac surgery.7-10
The analyses were based on 11 736 consecutive patients undergoing coronary artery bypass grafting (CABG), heart valve surgery, or both procedures, between 1 June 2001 and 31 January 2006, at public hospitals in Victoria. The six hospitals involved in data collection for the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) Victorian Cardiac Surgery Database Project11 were the Royal Melbourne Hospital, the Alfred Hospital, Monash Medical Centre, Geelong Hospital, Austin and Repatriation Medical Centre, and St Vincent’s Hospital Melbourne. Data were collected prospectively for all patients as part of clinical care and follow-up by surgeons, perfusionists, resident medical officers and database managers. Data on 30-day mortality were obtained by telephone contact with patients, family members or medical practitioners. Patients undergoing other cardiac procedures concomitantly (eg, atrial septal defect closure or atrial arrhythmia surgery) or non-cardiac procedures (eg, related to the thoracic aorta) were excluded. The quality of the data is good, as participating surgical units are subject to regular audits and checks. Only 16 patients (0.14%) were excluded from the analysis because of missing data.
BMI, which defines the degree of obesity, was calculated using the height and weight at the time of surgery according to National Health and Nutrition Examination Survey criteria.12 Obesity was defined as BMI (kg/m2) > 30 to < 40. Morbid obesity was defined as BMI ≥ 40.12 We defined the normal weight group (BMI, 20–30) as the reference group. Underweight patients were those with a BMI < 20. We divided the study cohort into these four categories; the underweight group was excluded from the outcomes analysis.
Hypercholesterolaemia — history of a fasting cholesterol level > 5.0 mmol/L or treatment for hypercholesterolaemia.
Hypertension — blood pressure level > 140/90 mmHg or a history of high blood pressure, or taking anti-hypertensive medications.
Renal failure — last preoperative serum creatinine level > 200 μmol/L or dialysis-dependence before operation.
Cerebrovascular disease — any prior coma with the patient unresponsive for > 24 h, stroke or transient ischaemic attack, or carotid stenosis > 75%.
Peripheral vascular disease — any of: claudication, amputation for arterial insufficiency, reconstruction for aortoiliac occlusive disease or surgery for peripheral vascular disease, or documented abdominal aortic aneurysm.
Active valve endocarditis — valvular disease of infectious aetiology with positive blood culture or confirmation of endocarditis at operation, and receiving treatment for endocarditis at the time of surgery.
Twelve postoperative adverse outcomes were analysed:
Operative mortality — death ≤ 30 days after operation or > 30 days if still in hospital;
Stroke — new neurological deficit or coma > 24 h after operation;
Postoperative myocardial infarction — at least two of: cardiac enzyme level elevation, new cardiac wall motion abnormalities, or new Q waves on serial electrocardiograms;
Pneumonia — diagnosed by positive sputum cultures and consistent with clinical findings;
Prolonged ventilation — > 24 h;
Reintubation;
New occurrence of renal failure — at least two of the following: raised serum creatinine level > 200 μmol/L, increase in creatinine level (doubling or greater) compared with preoperative value, new requirement for dialysis or haemofiltration;
Deep sternal wound infection — involving muscle and bone (as shown by surgical exploration) and one of the following: positive cultures or treatment with antibiotics;
Readmission to the intensive care unit (ICU);
Return to theatre for any cause;
Return to theatre for bleeding;
Prolonged length of stay — > 14 days.
Continuous variables are presented as mean ± 1 SD. The Mann–Whitney U test was used to compare two groups of continuous variables. The χ2 test was used to compare groups of categorical variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using standard statistical methods. The prevalence of obesity in the study cohort was compared with the expected prevalence of obesity in the adult Australian population (age- and sex-specific data obtained from the AusDiab 1999–2000 study).3
Multivariate logistic regression analysis was used to examine the effect of obesity category on early morbidity and mortality, while adjusting for potentially confounding patient and operative variables.13 We adjusted for the variables that differed in prevalence between the study groups, and those that have previously been shown to affect short-term outcomes.14-16 These variables were: age, sex, diabetes, hypercholesterolaemia, hypertension, renal failure, cerebrovascular disease, peripheral vascular disease, lung disease, heart failure (New York Heart Association Class IV), severe left ventricular impairment (ejection fraction < 30%), emergency status, active infective endocarditis, previous cardiac surgery, operation type other than isolated coronary artery bypass grafting, and total cardiopulmonary bypass time. All calculated P values were two-sided, and values < 0.05 were considered significant. Statistical analysis was performed using SPSS, version 14.0 (SPSS Inc, Chicago, Ill, USA).
Overall, 7893 (67.3%) of the patients were classified as having normal weight, 276 (2.3%) were underweight with a BMI < 20, 3353 (28.6%) were obese, and 214 (1.8%) were morbidly obese. Compared with the general adult Australian population, the study cohort had more obese patients in all age groups examined (Box 1). Overall, 30.4% of our study cohort were obese compared with an expected prevalence of 21.2% (P < 0.001). The prevalence of obesity was highest in the 45–54 years age group (37.7%), followed by the 35–44 years age group (37.5%).
Box 2 shows patient and operative characteristics by obesity categories. Compared with normal weight patients, patients with obesity were younger, more likely to be women, more likely to have comorbidities (diabetes, hypercholesterolaemia, hypertension, and lung disease), more likely to undergo isolated CABG, and less likely to undergo heart valve surgery. Patients with obesity were less likely to have severe left ventricular impairment or active infective endocarditis, or to undergo emergency surgery. Patients with morbid obesity were younger, more likely to be women, and to have diabetes, hypertension and lung disease, and less likely to have peripheral vascular disease. There were no differences between normal weight patients and those with obesity in the prevalence of preoperative renal failure, New York Heart Association Class IV status, previous cardiac surgery or bilateral internal mammary artery use, and cardiopulmonary bypass times were similar.
The incidence of adverse postoperative outcomes is shown in Box 3, and the adjusted ORs for outcomes are shown in Box 4. There was no association between obesity or morbid obesity and operative mortality, stroke, postoperative myocardial infarction, pneumonia, reintubation, return to theatre for any cause, or return to theatre because of bleeding. Morbid obesity (but not obesity) was associated with prolonged ventilation (adjusted OR, 2.43), readmission to ICU (adjusted OR, 2.23) and prolonged length of stay (adjusted OR, 2.13). Both obesity and morbid obesity were associated with higher rates of new occurrence of renal failure (adjusted ORs, 1.38 and 2.90, respectively) and deep sternal wound infection (adjusted ORs, 2.38 and 7.24, respectively).
We also performed a subanalysis of the 9921 patients in our cohort who underwent CABG to determine the association between bilateral internal mammary artery use and deep sternal wound infection. Bilateral internal mammary artery use and obesity (as binary variables) were added to the 16 variables listed in Box 2 in a multivariate analysis. We found bilateral internal mammary artery use to be associated with a higher risk of deep sternal wound infection, with an adjusted OR of 3.14 (95% CI, 1.70–5.80).
We found that there were 1.4 times more patients with obesity undergoing CABG or heart valve surgery than would be expected for the general adult Australian population. The high prevalence of obesity in the study cohort is in keeping with recent data from the United Kingdom, where 27% of patients undergoing isolated CABG in 1999–2003 were obese.17 These figures are also similar to data from Europe and the United States,7,10,18,19 indicating that this trend is evident in most developed nations. Any comparisons between our findings and those of other studies must take into account differences in obesity definitions used. We elected to define obesity as a BMI over 30 to be consistent with the majority of the health science literature. However, other studies have used a defined percentile of the BMI of the study cohort (eg, highest 25% of BMI defined as obese),10 or a BMI more than 3518 as the cut-off for obesity.
We found obesity to be most prevalent in the younger age groups (35–54 years), at odds with Australian population data. In the AusDiab data, the two age groups with the highest prevalence of obesity were 55–64 years and 65–74 years.3 The higher prevalence of obesity in younger patients undergoing cardiac surgery may be due to referral bias. Clinicians may be less likely to consider patients who are obese, elderly and have comorbidities (the number of comorbidities also rises with age) as good candidates for surgery. Conversely, younger patients with obesity, as well as probably having fewer additional comorbidities than their older counterparts, are seen to have more to gain from the survival advantages conferred by cardiac surgery versus non-surgical therapies.
The adjusted early mortality rate was similar in patients who were obese or morbidly obese compared with the normal weight cohort, consistent with most of the recent studies of cardiac surgery looking at this issue.7-10,19 However, one study published in 2002 used the North American Society of Thoracic Surgeons’ database — the largest cardiac surgery database in existence.18 An analysis of a cohort of 590 000 patients showed a modest increase in operative mortality (adjusted OR, 1.21) in their moderate obesity group (BMI, 35–39.9), and a markedly higher risk of operative mortality (adjusted OR, 1.58) in their extreme obesity group (BMI, ≥ 40).18 The large size of the Society of Thoracic Surgeons’ database, and the different cut-offs for defining obesity, may account for the differences in findings.
Our study found that obesity and morbid obesity were both associated with a higher risk of deep sternal wound infection, with adjusted ORs of 2.38 and 7.24, respectively. This association between obesity and deep sternal wound infection has been well documented.5,18 Bilateral internal mammary artery use is also a known risk factor for deep sternal wound infection. However, surgeons did not avoid using bilateral internal mammary arteries in obese patients as the rates of use were similar between the groups. The tendency to use bilateral internal mammary artery grafting in younger patients to improve long-term graft patency and patient survival may account for this. In our subanalysis, we found bilateral internal mammary artery use to be associated with a higher risk of deep sternal wound infection (adjusted OR, 3.14). Hence, the long-term survival benefits of bilateral internal mammary artery use need to be balanced with the increased short-term risk of deep sternal wound infection.
Obesity is related to unfavourable changes in pulmonary function. This may explain our finding in the morbidly obese group of an increased risk of prolonged ventilation (adjusted OR, 2.43), and partly explain the higher rates of readmission to ICU (adjusted OR, 2.23) and the prolonged length of stay (adjusted OR, 2.13). This is consistent with the findings of the Society of Thoracic Surgeons’ database study in which both moderate obesity (BMI, 35–39.9) and extreme obesity (BMI, ≥ 40) were associated with a higher risk of prolonged ventilation, with adjusted ORs of 1.49 and 1.70, respectively.18
Studies of the association between obesity and renal failure after cardiac surgery have given conflicting results.7-9,18,19 Our results document an increased risk of postoperative renal failure in both the obese and morbidly obese groups, although the reason is unclear. It is possible that the higher prevalence of hypertension and diabetes in obese patients may be associated with compromised preoperative renal function, which is exacerbated by the inflammatory response to cardiopulmonary bypass and cardiac surgery. There may be other as yet undiscovered mechanisms in obese patients that impair renal function above and beyond the known association of diabetes and hypertension. Population-based studies have found that obesity is independently associated with the development of chronic renal disease.20,21
1 Prevalence of obesity in the ASCTS database cohort compared with prevalence in age- and sex-matched adult Australians (AusDiab 2000, data adjusted for sex)3
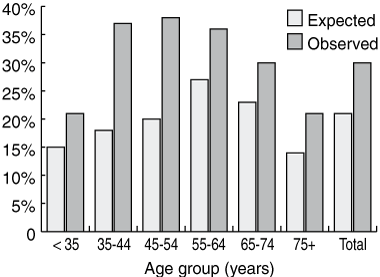
ASCTS = Australasian Society of Cardiac and Thoracic Surgeons. | |||||||||||||||
2 Patient and operative variables in patients undergoing cardiac surgery, by obesity category
Bilateral internal mammary artery use (of those having CABG) |
|||||||||||||||
3 Incidence of adverse outcomes after cardiac surgery, by obesity category
* χ2 test was used to determine significance. ICU = intensive care unit. |
|||||||||||||||
Received 31 October 2006, accepted 29 January 2007
- Cheng-Hon Yap1
- Morteza Mohajeri2
- Michael Yii1
- 1 St Vincent’s Hospital, University of Melbourne, Melbourne, VIC.
- 2 The Geelong Hospital, Geelong, VIC.
The Victorian Cardiac Surgery Database is an initiative of the Australasian Society of Cardiac and Thoracic Surgeons and is funded by the Department of Human Services, Victoria.
None identified.
- 1. Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med 1999; 341: 427-434.
- 2. Lafourtune G. Weighty problem. OECD Observer No. 238, July 2003. http://www.oecdobserver.org/news/fullstory.php/aid/1046/Weighty_problem.html (accessed Feb 2007).
- 3. Cameron AJ, Welborn TA, Zimmet PZ, et al. Overweight and obesity in Australia: the 1999–2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med J Aust 2003; 178: 427-432. <MJA full text>
- 4. Parsonnet V, Dean D, Bernstein AD. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation 1989; 79: I3-I12.
- 5. Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation 1999; 100: 1464-1480.
- 6. Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197-1209.
- 7. Engelman DT, Adams DH, Byrne JG, et al. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg 1999; 118: 866-873.
- 8. Kuduvalli M, Grayson AD, Oo AY, et al. Risk of morbidity and in-hospital mortality in obese patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg 2002; 22: 787-793.
- 9. Brandt M, Harder K, Walluscheck KP, et al. Severe obesity does not adversely affect perioperative mortality and morbidity in coronary artery bypass surgery. Eur J Cardiothorac Surg 2001; 19: 662-666.
- 10. Birkmeyer NJ, Charlesworth DC, Hernandez F, et al. Obesity and risk of adverse outcomes associated with coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation 1998; 97: 1689-1694.
- 11. Reid CM, Rockell M, Skillington PD, et al. Initial twelve months experience and analysis for 2001-2002 from the Australasian Society of Cardiac and Thoracic Surgeons — Victorian database project. Heart Lung Circ 2004; 13: 291-297.
- 12. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002; 288: 1723-1727.
- 13. Hosmer D, Lemeshow S. Applied logistic regression. New York: Wiley, 1989.
- 14. Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999; 16: 9-13.
- 15. Shroyer AL, Coombs LP, Peterson ED, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg 2003; 75: 1856-1864.
- 16. Rankin JS, Hammill BG, Ferguson TB Jr, et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg 2006; 131: 547-557.
- 17. Keogh B, Kinsman R. The Society of Cardiothoracic Surgeons of Great Britain and Ireland 5th National Adult Cardiac Surgical Database Report 2003. Henley-on-Thames: Dendrite Clinical Systems/SCTSBBI, 2004. http://www.scts.org/documents/PDF/5thBlueBook2003.pdf (accessed Feb 2007).
- 18. Prabhakar G, Haan CK, Peterson ED, et al. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons’ database. Ann Thorac Surg 2002; 74: 1125-1130.
- 19. Wigfield CH, Lindsey JD, Munoz A, et al. Is extreme obesity a risk factor for cardiac surgery? An analysis of patients with a BMI > or = 40. Eur J Cardiothorac Surg 2006; 29: 434-440.
- 20. Fox CS, Larson MG, Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. JAMA 2004; 291: 844-850.
- 21. Kramer H, Luke A, Bidani A, et al. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 2005; 46: 587-594.





Abstract
Objective: To assess the prevalence of obesity in patients undergoing coronary artery bypass grafting, heart valve surgery, or both procedures, and its association with postoperative outcomes.
Design and setting: Retrospective analysis of data, collected by the Australasian Society of Cardiac and Thoracic Surgeons Victorian Cardiac Surgery Database Project, on patients undergoing coronary artery bypass grafting, heart valve surgery, or both procedures, between 1 June 2001 and 31 January 2006.
Participants: 11 736 patients divided into four groups: underweight (body mass index [BMI], < 20), normal weight (BMI, 20–30), obese (BMI, > 30 to < 40), and morbidly obese (BMI, ≥ 40).
Main outcome measures: Prevalence of obesity (compared with the age- and sex-matched adult Australian population); associations between obesity and morbid obesity in cardiac patients and adverse postoperative outcomes.
Results: 30.4% of patients had a BMI > 30 (28.6% obese, 1.8% morbidly obese) compared with an expected prevalence of 21.2%. Morbid obesity was associated with prolonged ventilation (adjusted odds ratio [OR], 2.4; 95% CI, 1.6–3.7), readmission to intensive care (adjusted OR, 2.2; 95% CI, 1.2–4.1), and length of stay > 14 days (adjusted OR, 2.1; 95% CI, 1.4–3.3). Both obesity and morbid obesity were associated with renal failure (adjusted ORs, 1.4 [95% CI, 1.1–1.7] and 2.9 [95% CI, 1.7–4.9], respectively) and deep sternal wound infection (adjusted ORs, 2.4 [95% CI, 1.5–3.8] and 7.2 [95% CI, 2.8–18.7], respectively).
Conclusions: Obesity is 1.4 times more prevalent in patients having coronary artery bypass grafting or heart valve surgery in Victoria compared with the general adult Australian population. Both obesity and morbid obesity are associated with early morbidity, but not mortality, after operation.