Chronic obstructive pulmonary disease (COPD) is a common disorder affecting up to 2.6 million Australians, with affected patients occupying 1000 hospital beds daily in Australia.1 Management of the exacerbations that characterise COPD is described in several international guidelines. Common to these guidelines is the recommendation for controlled oxygen therapy to treat hypoxaemia.2-4 The COPDX Plan published in this Journal in 2003 recommends administering a fraction of inspired oxygen (Fio2) of 0.28 (about equal to 2 L per minute of oxygen via nasal prongs, or a Venturi mask set at an Fio2 of 0.28) or less until the arterial tension of respiratory gases is determined by arterial blood gas (ABG) sampling.3 This recommendation is based on the knowledge that increasing arterial oxygen tension in patients with hypercapnic respiratory failure causes deleterious changes to alveolar ventilation and gas exchange, resulting in worsening acidosis5,6 that, in turn, leads to higher morbidity and mortality.7,8
A study from the United Kingdom has shown that controlled oxygen delivery is not emphasised in ambulance and nursing staff education, and is often overlooked by emergency and intensive care physicians.9 Our anecdotal experience was similar, and we undertook an audit of patients presenting to our university teaching hospital with an acute exacerbation of COPD (AECOPD) to determine: (i) the proportion of patients who received care consistent with international guidelines; and (ii) whether not adhering to such guidelines resulted in differing outcomes for patients.
We undertook a retrospective audit of patients admitted after presenting to the emergency department (ED) with AECOPD. Patients discharged between 1 June and 30 September 2005 with a diagnosis of AECOPD were identified. Included patients had respiratory function test (RFT) results (either before or after admission) consistent with the British Thoracic Society (BTS) definition of COPD (ie, forced expiratory volume in 1 second [FEV1] less than 80% of predicted, and forced expiratory ratio less than 70%).4
One hundred potentially eligible patients were identified, and 35 of these were excluded (20 had not had RFTs, nine did not have RFT results consistent with BTS guidelines, and six were not admitted through the ED). The demographic characteristics of the remaining 65 patients are shown in Box 1.
ABG samples were taken from 43 patients (66%; 95% CI, 54.0%–77%) in the ED. All four patients in triage category 1 (100%; 95% CI, 48%–99%) had ABG tests. Corresponding numbers of patients in triage categories 2, 3 and 4 who had ABG tests were 11/19 (58%; 95% CI, 36%–77%), 21/31 (68%; 95% CI, 50%–81%) and 7/11 (64%; 95% CI, 35%–85%). Patients were triaged according to the Australasian Triage Scale.10
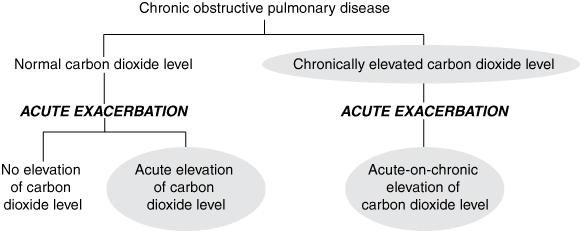
Thirty of the 65 patients presenting to the ED (46%; 95% CI, 35%–58%) had ABG results showing hypercapnia, either chron-ically (18 patients) or in association with respiratory acidosis (12 patients). Of these 30 patients with previously documented CO2 retention, 14 (47%) had ABG tests in the ED, all of which showed hypercapnia. An additional 11 patients (with no previous documentation of hypercapnia) were shown to have hypercapnia on ABG testing in the ED; this gave 25 ABG samples showing hypercapnia. A total of 41 patients had recorded hypercapnia (30 previously documented and 11 newly documented). These 41 patients were classified as retaining CO2 (see Box 2). Of note, 18 of the 65 patients presenting with COPD (28%; 95% CI, 18%–40%) had an ABG sample showing respiratory acidosis (pH < 7.35). Twenty-two patients (34%; 95% CI, 24%–46%) did not have an ABG test at any stage during their ED admission.
Of the 41 patients classified as retaining CO2, only two (5%; 95% CI, 2%–16%) received oxygen at a flow rate of 2 L per minute or less (≤ 2 L/min, as per COPDX guidelines3), while 13 (32%; 95% CI, 20%–47%) received oxygen at 4 L or less per minute (≤ 4 L/min, consistent with BTS guidelines4). Because of the small number of patients receiving oxygen at 2 L or less per minute, the group of patients retaining CO2 was further analysed by using an oxygen flow rate of 4 L per minute as a cut-off. Oxygen at 4 L per minute was delivered exclusively by means of nasal prongs. When flow rates of 5 L per minute or greater were used, the means of delivery was almost always a facemask, and when NIV was used, the Fio2 was set at 0.4 or higher. Results are shown in Box 3.
In the ED, patients with AECOPD are often treated with high-flow oxygen because of hypoxia. All COPD patients who had an ABG test were analysed in terms of highest achieved partial pressure of arterial oxygen (Pao2) to see if correcting hypoxia improved outcomes (Box 4). The median value for Pao2 was 74.5 mmHg. Patients were divided into those with Pao2 greater than or equal to, and less than 74.5 mmHg. The group with the higher Pao2 had significantly more patients in higher triage categories (P = 0.033), a longer stay in hospital (P = 0.029), greater need for NIV on admission (P = 0.0124) and a higher rate of admission to the high dependency unit (HDU; P = 0.0124). The multivariate linear regression model showed that pH (P = 0.012) and Pao2 (P = 0.012) were predictive of length of stay, while triage category and FEV1 were not. After adjusting for severity of exacerbation (ie, pH), Pao2 was a significant predictor of length of stay (P = 0.033). No factors were found to be significantly confounding the use of NIV or admission to the HDU.
Our findings confirm that uncontrolled oxygen therapy is commonplace at our university teaching hospital. The use of oxygen flow rates greater than 4 L per minute in patients with high CO2 levels was associated with a significant increase in length of stay (P = 0.034), a greater need for NIV (P = 0.033), and a higher admission rate to the HDU (P = 0.033). Use of more than 4 L of oxygen per minute was associated with severe acidosis in patients with AECOPD known to retain CO2 (P = 0.034). This association has been shown previously in a similar group of patients.9
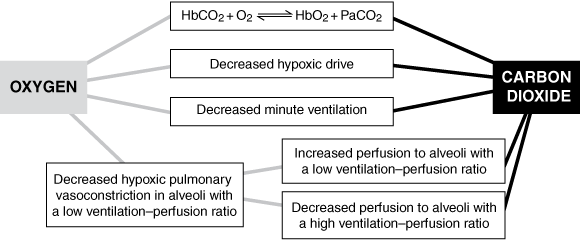
Increased oxygen delivery promotes retention of CO2 through numerous mechanisms (Box 5). The most important of these are generally considered to be an increase in ventilation–perfusion mismatch secondary to reduced hypoxic vasoconstriction11-13 and a reduction in ventilation on removal of a hypoxic stimulus.13,14
Our audit shows that the administration of high-flow oxygen usually begins in the ambulance. At the time of writing, no specific guideline exists for oxygen administration by ambulance officers.15 High-flow oxygen therapy is continued in the ED, where more than 80% of patients with AECOPD receive oxygen at more than 2 L per minute. This occurs in an ad-hoc fashion, with no formal method for prescribing oxygen. Furthermore, 22/65 patients receiving oxygen at more than 2 L per minute (34%; 95% CI, 24%–46%) did not have an ABG test. This highlights the need for more judicious use of ABG tests in monitoring patients who present with AECOPD at our university teaching hospital.
1 Demographic characteristics of the 65 patients included in the study
3 Comparison of the 41 patients who retained carbon dioxide on the basis of whether they received oxygen at > 4 L per minute or ≤ 4 L per minute
4 Comparison of patients with high and low partial pressure of arterial oxygen (Pao2)
- Simon A Joosten1
- Mariko S Koh2
- Xiaoning Bu3
- David Smallwood4
- Louis B Irving5
- Department of Respiratory Medicine, The Royal Melbourne Hospital, Melbourne, VIC.
We thank Anna Hutchinson, Michelle Thompson and Marcus Volz for their invaluable help with the statistical analysis and the Safety and Service Improvement Unit at The Royal Melbourne Hospital for their invaluable input.
None identified.
- 1. Australian Lung Foundation. Case statement: chronic obstructive pulmonary disease. Brisbane: ALF, 2001. http://www.lungnet.org.au/copd/copd_case_statements.html (accessed Nov 2006).
- 2. Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163: 1256-1276.
- 3. McKenzie DK, Frith PA, Burdon JGW, Town GI. The COPDX Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease 2003. Med J Aust 2003; 178 (6 Suppl): S1-S40. <MJA full text>
- 4. National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. London: National Institute for Clinical Excellence, 2004.
- 5. Plant PK, Owen JL, Elliot MW. One year period prevalence study of respiratory acidosis in acute exacerbation of COPD: implications for the provision of non-invasive ventilation and oxygen administration. Thorax 2000; 55: 550-554.
- 6. Donald KW. Neurological effects of oxygen. Lancet 1949; 2: 1056-1057.
- 7. Kettel LJ, Diener CF, Morse JO, et al. Treatment of respiratory acidosis in chronic obstructive lung disease. JAMA 1971; 217: 1503-1508.
- 8. Warren PM, Flenley DC, Millar JS, et al. Respiratory failure revisited: acute exacerbations of chronic bronchitis between 1961–68 and 1970–76. Lancet 1980; 1: 467-470.
- 9. Denniston AK, O’Brien C, Stableforth D. The use of oxygen in acute exacerbations of chronic obstructive pulmonary disease: a prospective audit of pre-hospital and hospital emergency management. Clin Med 2002; 2: 449-451.
- 10. Australasian College for Emergency Medicine. Guidelines for the implementation of the Australasian Triage Scale in emergency departments. G24. Melbourne: The College, November 2000. http://www.acem.org.au/infocentre.aspx?docId=59 (accessed Nov 2006).
- 11. Aubier M, Murciano D, Fournier M, et al. Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1980; 122: 191-199.
- 12. Sassoon CSH, Hassell KT, Mahutte CK. Hyperoxic-induced hypercapnia in stable chronic obstructive pulmonary disease. Am Rev Respir Dis 1987; 135: 907-911.
- 13. Robinson TD, Frieberg DB, Regnis JA, et al. The role of hypoventilation and ventilation-perfusion redistribution in oxygen-induced hypercapnia during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 1524-1529.
- 14. Dunn WF, Nelson SB, Hubmayr RD. Oxygen-induced hypercarbia in obstructive pulmonary disease. Am Rev Respir Dis 1991; 144 (3 Pt 1): 526-530.
- 15. Rural Ambulance Victoria. Clinical practice guidelines. (Updated Feb 2005.) http://www.rav.vic.gov.au (accessed Feb 2007).





Abstract
Objective: To elucidate oxygen administration practices in the setting of acute exacerbations of chronic obstructive pulmonary disease (COPD) and compare these practices with those recommended in internationally accepted guidelines.
Design: Retrospective audit.
Participants and setting: 65 patients admitted to a Melbourne university teaching hospital via the emergency department (ED), identified through medical records by a discharge diagnosis (discharged between 1 June and 30 September 2005) of acute exacerbation of COPD (AECOPD). Those included had respiratory function test results consistent with British Thoracic Society guidelines for the diagnosis of COPD.
Main outcome measures: Length of stay, need for high dependency unit (HDU) admission, use of non-invasive ventilation (NIV), and use of arterial blood gas (ABG) tests.
Results: Our audit showed that 95% of patients defined as retaining carbon dioxide received oxygen at a flow rate greater than 2 L/min. This process began in the ambulance and continued in the ED, often without monitoring of ABG levels. Length of stay was significantly longer (P = 0.029); need for NIV on admission greater (P = 0.0124); and rate of admission to the HDU higher (P = 0.0124) in patients who achieved a partial pressure of arterial oxygen (Pao2) ≥ 74.5 mmHg compared with those with a Pao2 < 74.5 mmHg.
Conclusions: The vast majority of patients with AECOPD presenting to our university teaching hospital receive oxygen therapy outside of internationally accepted guidelines, often without monitoring of ABG levels. The use of high-flow oxygen may contribute to an increased length of stay, more frequent admission to HDU and greater use of NIV among patients who achieve a higher Pao2.