Over the past two decades, a resurgence of invasive group A streptococcal (GAS) infection has been observed in industrialised countries around the world. This has been attributed to the emergence of highly virulent strains of group A streptococcus.1,2 With current treatments, mortality still approaches 30% and survivors may be left with chronic sequelae.3
Little is known about the epidemiology of invasive GAS infection in Australia. A recent study in northern Queensland reported an annual incidence rate of 10.3 per 100 000 in the non-Indigenous population and 82.5 per 100 000 in the Indigenous population, with an overall mortality rate of 7%.4 These estimates are substantially higher than those reported from the Top End of the Northern Territory (6.4 per 100 000 in the non-Indigenous population and 32.2 per 100 000 in the Indigenous population).5 The only data from non-tropical Australia came from a 12-year review of admissions to the Royal Children’s Hospital in Melbourne, which identified a clear increase in both disease frequency and severity,6 consistent with reports from the United States and Europe.
New approaches to the management of invasive infections have emerged, including the use of clindamycin in addition to penicillin and the use of intravenous immune globulin (IVIG) in the treatment of streptococcal toxic shock syndrome (STSS).7-11 After decades of research, prophylactic candidate GAS vaccines are beginning to show promise and may be available within the next 10 years.12-17 To evaluate these interventions, comprehensive baseline data on the burden of disease are critical. Therefore, we established intensive, active surveillance of invasive GAS infection in Victoria.
Confirmed cases were defined as one of the following:
the isolation of group A streptococcus from a normally sterile site (eg, blood, cerebrospinal fluid, or other sterile fluid or tissue);
a clinical presentation of necrotising fasciitis with evidence of GAS infection (eg, culture of group A streptococcus or the presence of gram-positive cocci from a tissue specimen or wound swab or positive streptococcal serology);
a clinical presentation of pharyngeal abscess (quinsy) requiring hospitalisation and parenteral antibiotics with the isolation of group A streptococcus from a throat swab.
STSS was defined according to published criteria (Box 1).18 Infections were classified as nosocomially acquired if the collection date of the first positive specimen was at least 3 days after the hospital admission date.
Demographic and clinical data were collected from the treating physician and medical record review.
Available isolates underwent emm sequence typing and penicillin susceptibility testing. A subset of 50 randomly selected isolates (sample limited because of cost) underwent testing to determine minimal inhibitory concentrations (MIC) to penicillin, clindamycin and erythromycin using the Etest method (AB Biodisk, Solna, Sweden). Susceptibility was assigned according to the interpretative criteria for MIC recommended by the Clinical and Laboratory Standards Institute.19
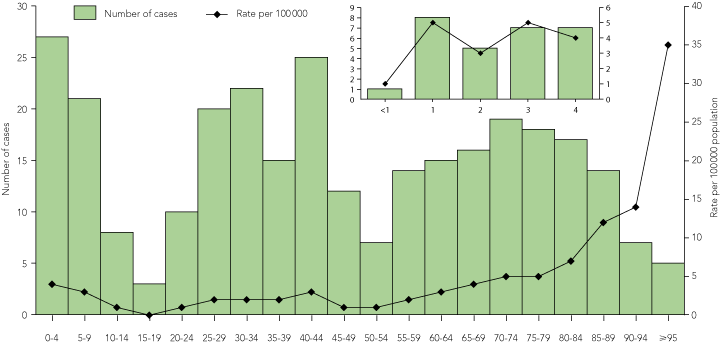
For the 302 patients whose age was known, the mean age was 45.8 years (95% CI, 42.6–49.0 years); 58 cases (19.2%) occurred in children aged 0–15 years and 134 (44.4%) occurred in people aged 50 years and older. Incidence rates were highest in people younger than 5 years and in those 65 years and older (Box 2). Fifty-three per cent of cases occurred in males. Only two cases were identified in Indigenous individuals (average annual incidence rate, 2.9 per 100 000 population per year).
Soft tissue was the most common site of infection (n = 176) across all age groups (Box 3). Among the patients for whom the information was available, a pre-existing chronic medical condition was reported in 66.2% (182/275); heart and lung diseases predominated. Immunosuppression was reported in 18.7% of patients (52/278), and 23.8% (62/261) reported use of non-steroidal anti-inflammatory drugs within 14 days of symptom onset. Preceding chickenpox was reported in 5% (3/58) of paediatric patients. Other risk factors are listed in Box 4.
STSS was identified in 48 (14.4%) of the 333 patients, and necrotising fasciitis in 30 (10.9%) of 276 patients for whom the information was available (Box 5). Twenty-five deaths were known to have been due to GAS disease (case-fatality rate, 7.8%). Six deaths (24%) occurred in people younger than 65 years, including a 2-year-old child with a history of sore throat who died of streptococcal bacteraemia. All but three died within 10 days of admission to hospital. Of those who died, only one person had received IVIG and only eight had received clindamycin in hospital. Four people who died were immunocompromised; none was HIV positive. The focus of infection in 19 fatal cases (76%) was soft tissue, of which five involved necrotising fasciitis.
Among the 255 available isolates on which emm sequence typing was performed, 56 different types were identified; the top five are presented in Box 6. Type 1 was the most common in all age groups, and accounted for 42% of isolates from those who died, and 47% of STSS and 70% of necrotising fasciitis cases. Sixty-two per cent of the isolates came from emm types included in a 26-valent serotype-specific GAS vaccine currently in clinical trials.17 Emm types were available on 15 of the 21 nosocomial infections: three were type 1, two were type 12, two were type 28, and eight were individual types. No epidemiological links were apparent.
The incidence of disease, case-fatality rate, predominance of cases in the elderly and people with underlying medical conditions, foci of infection and frequency of emm type 1 among invasive isolates are consistent with epidemiological data from other industrialised countries.20-23 The incidence of invasive GAS infection in non-Indigenous people in tropical northern Australia is two to four times greater than in Victoria.4,5 It is not clear if these differences are attributable to factors related to host susceptibility (eg, increased prevalence of chronic conditions and alcohol use), differences in environmental exposures, or differences in the virulence of prevalent GAS strains. The differences in emm type distribution between northern Australia and Victoria reflect their divergent epidemiologies. Cases in the north are largely due to underlying skin infections,5 and the extreme diversity in emm types in invasive GAS disease reflects the same diversity in skin strains.5,24 The limited emm type distribution in Victoria parallels that seen in other industrialised countries.
The proportion of infections that were nosocomially acquired is of concern. Invasive GAS infection in health care settings can be highly transmissible25 and is associated with an increased risk of a fatal outcome;26,27 institutional outbreaks are not uncommon.28 In the US, it is recommended that one nosocomial postpartum or postsurgical invasive GAS infection should initiate enhanced surveillance and two or more should prompt an epidemiological investigation that includes carriage studies of health care workers.29 Our data suggest these recommendations are prudent and should be considered in Australia.
We found a low proportion of varicella-associated childhood cases compared with between 15% and 27% of paediatric cases reported elsewhere.30-32 The reasons for this are unclear. Hospitalisation for childhood varicella in Victoria occurs relatively late in the illness,33 suggesting that the low rate of varicella-associated invasive GAS infections is not due to different behaviour in seeking health care. However, at the time of this study there was considerable public concern surrounding invasive meningococcal disease in Australia; it is possible that this may have led parents of children with fever and rash to present early to primary care practitioners.
Under-reporting is usually the most common problem with the use of surveillance systems for providing burden of disease data. Our system of reviewing hospital data and regular contact with diagnostic laboratories suggests we are not likely to have missed many confirmed cases. We included in our case definition 18 cases that were not confirmed by the isolation of group A streptococcus from a sterile site. One of these was a patient with necrotising fasciitis who had both positive streptococcal serology and group A streptococcus isolated from a deep wound swab, and developed STSS. The other 17 had pharyngeal abscesses (quinsy), which are accepted invasive infections for which the most common single pathogenic species is group A streptococcus;34 all these patients had positive throat swabs. Both conditions require management as intensive as for group A streptococcus confirmed by sterile site isolates, and their exclusion would underestimate disease burden.
The incidence of invasive GAS disease in Victoria is similar to that of invasive meningococcal disease before the introduction of meningococcal C vaccine.35 Invasive meningococcal disease has been legally notifiable in all parts of Australia since 1991, with comprehensive treatment and management guidelines developed at a national level.35 Given the similarities, there is a case for making invasive GAS infection notifiable in Australia. This would be strengthened if an increased risk of disease could be demonstrated in close contacts of cases; we are analysing our data to determine if this is the case in Victoria. The frequency of nosocomial infections provides impetus for the development of guidelines to manage GAS disease in institutions. The lack of use of IVIG or clindamycin in fatal cases, despite recommendations that they be used in patients with severe invasive GAS infections,36-38 suggests a need for standard treatment guidelines and education of health professionals. A review of the approved indications for IVIG in Victoria is required, as these do not currently include severe GAS disease, potentially delaying the release of the product for immediate use. The ongoing development of vaccines, concerns about antibiotic resistance and a growing body of evidence that suggests disease is becoming more severe in recent decades necessitate ongoing population-based surveillance systems to monitor these trends and to inform both clinical management and public health policy.
1 Case definition of streptococcal toxic shock syndrome
A. Isolation of group A streptococcus
2. From a non-sterile body site
2. Two or more of the following clinical or laboratory abnormalities:
d. Acute respiratory distress syndrome
2 Confirmed invasive group A streptococcal infection and average annualised incidence rate per 100 000 by age group (years), Victoria, 1 March 2002 to 31 August 2004
 | |||||||||||||||
3 Specified diagnosis* by age group in people with confirmed invasive group A streptococcal infection, Victoria, 1 March 2002 to 31 August 2004
4 Frequency of known risk factors for confirmed invasive group A streptococcal infection, Victoria, 1 March 2002 to 31 August 2004
Received 25 August 2006, accepted 14 March 2007
- Kerry-Ann F O’Grady1,2
- Loraine Kelpie1,2
- Ross M Andrews3
- Nigel Curtis1,4,2
- Terence M Nolan1,2
- Gowri Selvaraj2
- Jonathan W Passmore5
- Frances Oppedisano2
- John A Carnie6
- Jonathan R Carapetis3
- 1 University of Melbourne, Melbourne, VIC.
- 2 Murdoch Children’s Research Institute, Melbourne, VIC.
- 3 Menzies School of Health Research, Charles Darwin University, Darwin, NT.
- 4 Royal Children’s Hospital, Melbourne, VIC.
- 5 Road Safety Major Projects, Transport Accident Commission, Melbourne, VIC.
- 6 Victorian Department of Human Services, Melbourne, VIC.
We would like to thank Dr Geoffrey Hogg and staff of the Microbiological Diagnostic Unit of the University of Melbourne, and all participating hospitals, doctors and laboratories for their assistance with this project. This work was funded by project grants from the National Health and Medical Research Council and the Victorian Department of Human Services. Jonathan Carapetis was supported by a career development award from the National Health and Medical Research Council.
None identified.
- 1. Stevens DL. Invasive group A streptococcus infections. Clin Infect Dis 1992; 14: 2-11.
- 2. Stevens DL, Tanner MH, Winship J, et al. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 1989; 321: 1-7.
- 3. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis 1995; 1: 69-78.
- 4. Norton R, Smith HV, Wood N, et al. Invasive group A streptococcal disease in North Queensland (1996–2001). Indian J Med Res 2004; 119 Suppl: 148-151.
- 5. Carapetis JR, Walker AM, Hibble M, et al. Clinical and epidemiological features of group A streptococcal bacteraemia in a region with hyperendemic superficial streptococcal infection. Epidemiol Infect 1999; 122: 59-65.
- 6. Carapetis J, Robins-Browne R, Martin D, et al. Increasing severity of invasive group A streptococcal disease in Australia: clinical and molecular epidemiological features and identification of a new virulent M-nontypeable clone. Clin Infect Dis 1995; 21: 1220-1227.
- 7. Norrby-Teglund A, Kaul R, Low DE, et al. Plasma from patients with severe invasive group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J Immunol 1996; 156: 3057-3064.
- 8. Basma H, Norrby-Teglund A, McGeer A, et al. Opsonic antibodies to the surface M protein of group A streptococci in pooled normal immunoglobulins (IVIG): potential impact on the clinical efficacy of IVIG therapy for severe invasive group A streptococcal infections. Infect Immun 1998; 66: 2279-2283.
- 9. Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome — a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis 1999; 28: 800-807.
- 10. Mascini EM, Jansze M, Schouls LM, et al. Penicillin and clindamycin differentially inhibit the production of pyrogenic exotoxins A and B by group A streptococci. Int J Antimicrob Agents 2001; 18: 395-398.
- 11. Darenberg J, Ihendyane N, Sjolin J, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003; 37: 333-340.
- 12. Dale JB, Penfound T, Chiang EY, et al. Multivalent group A streptococcal vaccine elicits bactericidal antibodies against variant M subtypes. Clin Diagn Lab Immunol 2005; 12: 833-836.
- 13. Bisno AL, Rubin FA, Cleary PP, Dale JB. Prospects for a group A streptococcal vaccine: rationale, feasibility, and obstacles — report of a National Institute of Allergy and Infectious Diseases workshop. Clin Infect Dis 2005; 41: 1150-1156.
- 14. Olive C, Hsien K, Horvath A, et al. Protection against group A streptococcal infection by vaccination with self-adjuvanting lipid core M protein peptides. Vaccine 2005; 23: 2298-2303.
- 15. McMillan DJ, Chhatwal GS. Prospects for a group A streptococcal vaccine. Curr Opin Mol Ther 2005; 7: 11-16.
- 16. Pichichero ME. Group A streptococcal vaccines. JAMA 2004; 292: 738-739.
- 17. McNeil SA, Halperin SA, Langley JM, et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin Infect Dis 2005; 41: 1114-1122.
- 18. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. The Working Group on Severe Streptococcal Infections. JAMA 1993; 269: 390-391.
- 19. Clinical and Laboratory Standards Institute. Performance of standards for antimicrobial susceptibility testing; sixteenth informational supplement (CLSI document M100-S16). Wayne, Pa: CLSI, 2006.
- 20. Al Mazrou AM. Group A streptococcal bacteraemia: experience at a university hospital in Riyadh. J Infect 1997; 34: 95-100.
- 21. Andersen MM, Ronne T. Group A streptococcal bacteraemias in Denmark 1987–89. J Infect 1995; 31: 33-37.
- 22. Chiobotaru P, Yagupsky P, Fraser D, Dagan R. Changing epidemiology of invasive Streptococcus pyogenes infections in southern Israel: differences between two ethnic population groups. Pediatr Infect Dis J 1997; 16: 195-199.
- 23. Zurawski CA, Bardsley M, Beall B, et al. Invasive group A streptococcal disease in metropolitan Atlanta: a population-based assessment. Clin Infect Dis 1998; 27: 150-157.
- 24. Bessen D, Carapetis J, Beall B, et al. Contrasting molecular epidemiology of group A streptococcus causing tropical and non-tropical infections of the skin and throat. J Infect Dis 2000; 182: 1109-1116.
- 25. Kakis A, Gibbs L, Eguia J, et al. An outbreak of group A streptococcal infection among health care workers. Clin Infect Dis 2002; 35: 1353-1359.
- 26. Bernaldo de Quiros JC, Moreno S, Cercenado E, et al. Group A streptococcal bacteremia: a 10 year prospective study. Medicine (Baltimore) 1997; 76: 238-248.
- 27. Ramage L, Green K, Pyskir D, Simor AE. An outbreak of fatal nosocomial infections due to group A streptococcus on a medical ward. Infect Control Hosp Epidemiol 1996; 17: 429-431.
- 28. Weber DJ, Rutala WA, Denny FWJ. Management of healthcare workers with pharyngitis or suspected streptococcal infections. Infect Control Hosp Epidemiol 1996; 17: 753-761.
- 29. Centers for Disease Control and Prevention. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35: 950-959.
- 30. Abuhammour A, Hasan RA, Unuvar E. Group A beta-hemolytic streptococcal bacteremia. Indian J Pediatr 2004; 71: 915-919.
- 31. Laupland KB, Davies D, Low DE, et al. Invasive group A streptococcal disease in children and association with varicella-zoster virus infection. Pediatrics 2000; 105: e60.
- 32. Tyrrell GJ, Lovgren M, Kress B, Grimsrud K. Invasive group A streptococcal disease in Alberta, Canada (2000 to 2002). J Clin Microbiol 2005; 43: 1678-1683.
- 33. Carapetis JR, Russell DM, Curtis N. The burden and cost of hospitalised varicella and zoster in Australian children. Vaccine 2004; 23: 755-761.
- 34. Jousimies-Solmer H, Savolainen S, Makitie A, Ylikoski J. Bacteriologic findings in peritonsillar abscesses in young adults. Clin Infect Dis 1993; 16 Suppl 4: S292-S298.
- 35. Communicable Diseases Network Australia. Guidelines for the early clinical and public health management of meningococcal disease in Australia. Canberra: Commonwealth Department of Health and Ageing, 2001. http://www.health.gov.au/internet/wcms/publishing.nsf/Content/cda-pubs-other-mening.htm (accessed Apr 2007).
- 36. Norrby-Teglund A, Muller MP, McGeer A, et al. Successful management of severe group A streptococcal soft tissue infections using an aggressive medical regimen including intravenous polyspecific immunoglobulin together with a conservative surgical approach. Scand J Infect Dis 2005; 37: 166-172.
- 37. American Academy of Pediatrics Committee on Infectious Diseases. Severe invasive group A streptococcal infections: a subject review. Pediatrics 1998; 101: 136-140.
- 38. Mulla ZD. Treatment options in the management of necrotising fasciitis caused by group A streptococcus. Expert Opin Pharmacother 2004; 5: 1695-1700.





Abstract
Objective: To estimate the incidence and severity of invasive group A streptococcal infection in Victoria, Australia.
Design: Prospective active surveillance study.
Setting: Public and private laboratories, hospitals and general practitioners throughout Victoria.
Patients: People in Victoria diagnosed with group A streptococcal disease notified to the surveillance system between 1 March 2002 and 31 August 2004.
Main outcome measure: Confirmed invasive group A streptococcal disease.
Results: We identified 333 confirmed cases: an average annualised incidence rate of 2.7 (95% CI, 2.3–3.2) per 100 000 population per year. Rates were highest in people aged 65 years and older and those younger than 5 years. The case-fatality rate was 7.8%. Streptococcal toxic shock syndrome occurred in 48 patients (14.4%), with a case-fatality rate of 23%. Thirty cases of necrotising fasciitis were reported; five (17%) of these patients died. Type 1 (23%) was the most frequently identified emm sequence type in all age groups. All tested isolates were susceptible to penicillin and clindamycin. Two isolates (4%) were resistant to erythromycin.
Conclusion: The incidence of invasive group A streptococcal disease in temperate Australia is greater than previously appreciated and warrants greater public health attention, including its designation as a notifiable disease.