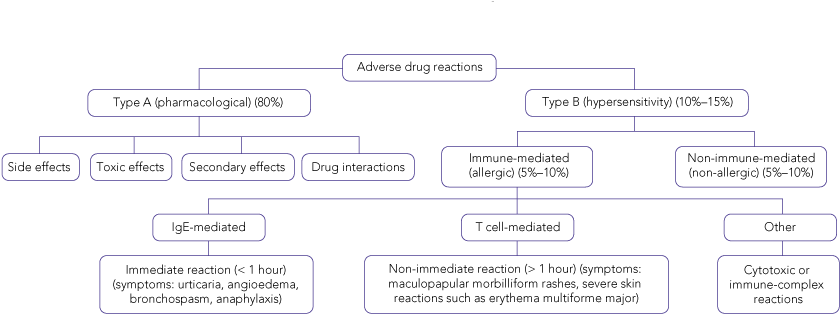
The World Health Organization defines an ADR as a response to a drug that is noxious, unintended or undesired occurring at doses normally used for the prevention, diagnosis or treatment of disease.1 A pharmacological classification2 divides most ADRs into one of two major subtypes: type A and type B reactions.
Type B reactions are hypersensitivity reactions that are unpredictable and not dose-dependent. They lead to objectively reproducible symptoms or signs at a dose tolerated by normal people.3 Type B reactions comprise about 10%–15% of all ADRs. Drug allergies, which comprise 5%–10% of ADRs,4 are hypersensitivity reactions that involve an immune mechanism (IgE- or T cell-mediated, or, rarely, involving an immune complex or cytotoxic reaction). All other hypersensitivity drug reactions without an immune mechanism (5%–10%) — or in which an immunological process is not proven — are classified as non-immune (or non-allergic) hypersensitivity reactions (Box 1).3
What was the temporal relationship between ingestion/administration of the drug and onset of the reaction?
Was the nature of the reaction in keeping with known adverse reactions to the drug?
Did the reaction resolve with cessation of the drug, and did it recur if the drug was recommenced?
Were other drugs administered concurrently that could have caused the reaction?
Was/Were there any underlying condition(s) of the patient that could explain the reaction?
The pharmacological features of type A adverse reactions (toxicity, side effects, secondary effects and drug interactions) can often be determined by searches of pharmacological references and databases such as the Australian medicines handbook5 or MIMS annual.6 However, doctors are sometimes faced with an unclear history or lack of supportive documentation, making this important part of the evaluation indeterminate.
Did the observed reaction occur on first exposure to the drug?
What was the nature of the reaction?
What was the time course of the reaction?
Urticaria, angioedema, bronchospasm and anaphylaxis signify mast cell activation, and usually indicate an immune mechanism mediated by drug-specific IgE antibodies (Box 2). However, some drugs (eg, radiocontrast media, aspirin or vancomycin) may activate mast cells directly, or through non-immune mechanisms, without previous exposure (Box 3).
Life-threatening conditions such as erythema multiforme major (Stevens–Johnson syndrome) and toxic epidermal necrolysis (Box 4), which cause widespread desquamation, mucosal ulceration and high fevers, are also mediated by T cells. The effector cells in these reactions are drug-specific memory T cells, but the exact mechanism is unclear.
Non-immediate reactions (occurring more than 1 hour after drug administration) suggest a drug-specific T cell-mediated mechanism.7 These late reactions may present in a variety of ways, including fixed drug eruptions, maculopapular morbilliform rashes, and bullous or pustular exanthems. Other non-cutaneous manifestations may include unexplained pyrexias, arthralgia, myalgia, eosinophilia or other haematological abnormalities, and derangement of liver function. These non-immediate reactions are not IgE-mediated, which has implications for diagnostic testing and management.
Skin testing (by skin prick or intradermally) is of predictive value for only a limited group of IgE-mediated drug allergic reactions. The best characterised drug is penicillin, for which the immunogenic isotopes (the parts of the molecule recognised by the immune system) have been identified. They consist of a major determinant (accounting for 95% of penicillin degradation metabolites) and minor determinants (accounting for 5% of metabolites). Testing with major and minor determinants is done with a skin prick test, followed by an intradermal test if the skin prick test is negative. (The commercial product Pre-Pen [Hollister-Stier], which contains the major determinant of penicillin, is currently unavailable anywhere, but alternatives may be available in the near future.) A weal diameter of at least 3 mm greater than that of the negative control, together with erythema, constitutes a positive test. US studies have shown that a negative test in patients with an indeterminate clinical history indicates that penicillin can be administered with less than 4% risk of an immediate reaction8 (similar to the risk in the general population). However, more recent European research indicates that the predictive value is much lower in patients with a documented high probability clinical history of immediate reaction.9
Amoxycillin and ampicillin should be included in the skin test array to improve the diagnostic value.10 This is because, with changes in drug prescription patterns, some patients are developing immunological reactivity to β-lactam sidechains specific to amoxycillin and ampicillin molecules, rather than the classical major and minor determinants common to all synthetic penicillin β-lactams.
Other drugs for which skin prick and intradermal tests are of predictive value include muscle relaxants, insulin, and biological agents such as Gelofusine (B. Braun) (a plasma volume expander) and streptokinase. Patch tests are done by making a 5% concentration of the relevant drug in a vehicle such as petrolatum, applying it to the skin and measuring the reaction after 48–72 hours. They are used to study non-immediate reactions, although their clinical diagnostic value is limited.11 However, for the vast majority of allergic drug reactions, there are no validated skin tests that have been shown to be of predictive value. This is because the reactions are either not IgE-mediated, or the relevant immunogenic epitopes (which may be derived from unidentified drug metabolites or breakdown products) have not been identified for most drugs.
The radioallergosorbent test (RAST) and the non-radioactive enzyme-linked immunosorbent assay (ELISA) are commercially available in-vitro tests for detecting serum specific IgE antibodies. The tests are only available for some β-lactam antibiotics, as the immunogenic epitopes for most drugs are unknown. Like other in-vitro tests, they are generally more specific but less sensitive than skin tests. Hence, they have poor negative predictive values but better positive predictive values, and are used in conjunction with clinical evaluation and skin tests.10
The lymphocyte transformation test detects drug-specific T cells, which may be involved in some delayed allergic hypersensitivity reactions, but this is used for research purposes and has limited clinical application.12
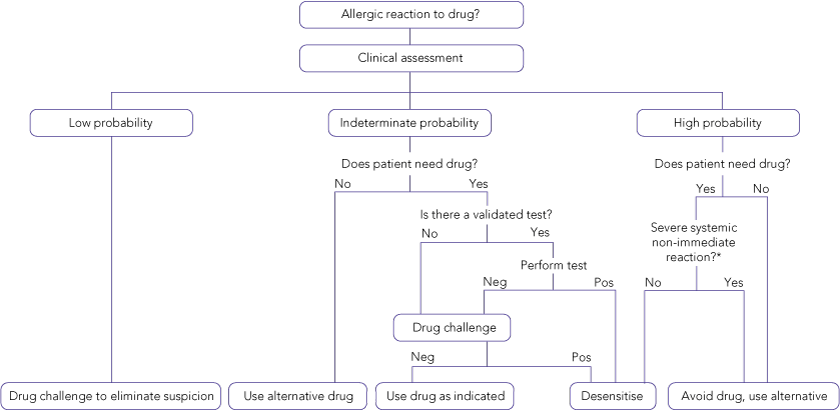
When the clinical evaluation suggests low or indeterminate clinical probability of a causal relationship between the drug and the ADR, the reaction is not severe, and skin or in-vitro tests are not available or helpful, a drug provocation challenge may be considered, to eliminate suspicion and allow the drug to be used in future;
When clinical evaluation suggests a moderate to high probability that a particular drug was the cause of an ADR and a safe alternative is required, another chemically or structurally different or unrelated drug may be given under monitored challenge to exclude the possibility of cross-reactivity between the two drugs;
When a drug is implicated with high probability (with or without supportive skin and in-vitro tests, if available) and is the drug of choice, a drug provocation challenge may be given to confirm the diagnosis, followed (if positive) by desensitisation (see below) and therapeutic administration of maintenance doses.
Provocation tests should be done in specialised clinics or hospitals with established protocols and resuscitation facilities, and should never be conducted in patients with a history of severe, life-threatening vasculitic syndromes, exfoliative dermatitis, erythema multiforme major, drug-induced hypersensitivity reactions with eosinophilia, or toxic epidermal necrolysis.13
The approach to the patient with a suspected ADR must be very methodical (Box 5). Firstly, as outlined above, a causal relationship must be established between the drug and the reaction. Then the reaction type must be determined, if possible.
For type B (hypersensitivity) drug reactions, several options may be considered. After severe or life-threatening reactions, the drug should not be re-administered. For less severe reactions, a drug provocation challenge may be considered. For type B immunologically-mediated (allergic) reactions, the management option depends on the mechanism responsible for the reaction. If validated confirmatory tests are available, they should be used to determine the allergic status of the patient (eg, tests for penicillin-specific IgE antibodies). If such tests are not available — and in most cases they are not — several approaches can be taken. The simplest approach is to avoid the drug if an alternative agent is available. If an alternative drug does not exist, a graded challenge with the implicated agent can be done if the previous reaction was not life-threatening and not consistent with an IgE-mediated reaction. However, if the medication is needed as the drug of choice, then desensitisation should be considered.14
Allergic reactions to β-lactam drugs are the most common group of type B hypersensitivity ADRs. The clinical, diagnostic and management approach to IgE-mediated immediate penicillin allergy has already been discussed (see “Detecting allergen-specific IgE: skin testing”, above).15 For the majority of reactions that produce non-immediate exanthems (and are probably mediated by T cells rather than IgE), the approach depends on the severity of the reaction and the potential need for penicillin-based therapy in the future.
Lifelong avoidance of a drug is necessary for the rare but severe reactions such as erythema multiforme major or toxic epidermal necrolysis. With more common morbilliform maculopapular exanthems, as seen when amoxycillin is administered concurrently with a viral infection (eg, infectious mononucleosis), the exact mechanism is not clear, but may be due to viral infection altering the immune status of the host. In this situation, the drug can be administered safely again once the viral infection has resolved,16 highlighting the critical role of taking a detailed clinical history and making a careful assessment.
When a patient with immediate penicillin allergy requires an alternative β-lactam drug, consideration can be given to prescribing a cephalosporin. A review of 11 studies of cephalosporin administration to patients with a history of penicillin allergy found cephalosporin reactions in 4.4% of patients with positive skin tests for penicillin.17 A practical approach is to ascertain whether the previous penicillin reaction was an immediate IgE-mediated allergy, and, if not, a graded challenge can be performed to determine whether the cephalosporin is a safe alternative. If the previous penicillin reaction was immediate, evaluation with penicillin skin testing should be done first.
Hypersensitivity reactions to aspirin and other NSAIDs may have a number of clinical manifestations. One clinical syndrome is late-onset asthma (onset age in the 30s or 40s) with nasal polyposis, and later development of aspirin hypersensitivity, with NSAID ingestion provoking asthma and rhinitis. This may affect 5%–10% of people with asthma. It involves a non-immune hypersensitivity mechanism of increased leukotriene production caused by inhibition of the cyclo-oxygenase-1 (COX-1) enzyme.18 Such people are sensitive to aspirin and all older non-specific NSAIDs, but tolerate specific COX-2 inhibitors (such as celecoxib). However, there are other clinical syndromes in which there is no history of asthma or rhinitis and the patient has an isolated sensitivity to a specific NSAID.19 This may have an immunological basis with a putative IgE mechanism. Clarifying the history and recognising the clinical patterns can allow specific provocation challenges to ascertain safe alternatives and prevent unnecessary avoidance of aspirin or other NSAIDs (see Box 6).
Fact or fiction – true or false?
1. The majority of drug allergies can be investigated by validated and predictive skin tests (T/F)
Angioedema is a well recognised adverse reaction that affects 0.1%–0.5% of patients taking ACE inhibitors.20 Angioedema can first appear anywhere from a few hours to 8 years after an ACE inhibitor is taken, with up to 20% of cases being life-threatening.20 The reaction involves a non-immune hypersensitivity mechanism caused by the accumulation of plasma kinins (such as bradykinin) as the result of inhibition (by ACE inhibitors) of the kininases that normally metabolise and inactivate bradykinin.21 If a patient taking an ACE inhibitor develops angioedema, the cause must be assumed to be the ACE inhibitor, and the drug should be ceased immediately (until such time as the drug is proven not to be the cause) (Box 7). Rare instances of angioedema have also been reported after taking angiotensin-receptor antagonists, but these reactions may not be mediated by bradykinin.
Delayed reactions, such as maculopapular exanthems, associated with cotrimoxazole are probably mediated by T-cell responses to reactive sulfonamide metabolites. There is increased frequency of these reactions in certain clinical situations (eg, they affect 20%–80% of patients infected with HIV, compared with 1%–3% of non-HIV-infected patients, possibly due to altered drug metabolism).10 Management is by avoidance, but desensitisation is possible in some cases.
Another common clinical problem is the putative cross-reactivity between sulfonamide antibiotics and other sulfonamide-derived drugs (eg, diuretics, sulfonylureas, celecoxib and sumatriptan). Patients may report that they are “allergic” to “sulfur antibiotics”. Although the true nature of their reaction may often be obscure, they are excluded from taking drugs in these groups because of concern about supposed cross-reactivity with their sulfonamide component. However, this is only a theoretical concern that has not been borne out in clinical practice, and need not necessarily exclude their use if clinically indicated.23 Furthermore, there is no relationship between sulfonamide allergy and intolerance to sulfite preservatives in food.
for early assessment of possible drug reactions when the mechanism of reaction is unclear;
for advice on distinguishing sulfur antibiotic reactions from risk of reaction to sulfur-containing non-antibiotics or dietary sulfites;
for assessment of severe reactions, such as toxic epidermal necrolysis;
when avoidance of a particular drug is not an option;
to help choose a suitable NSAID when a patient has reacted to a drug in this class; or
when considering desensitisation if an implicated medication is the drug of choice.
1 Classification of adverse drug reactions, including hypersensitivity and immune-mediated drug allergy
3 Immediate hypersensitivity urticarial rash secondary to vancomycin administration
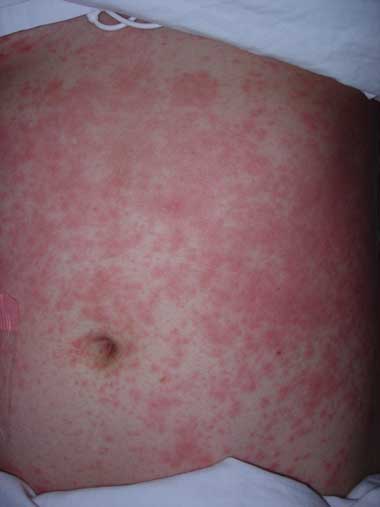
Courtesy of Department of Dermatology, Alfred Hospital, Melbourne.
4 Toxic epidermal necrolysis secondary to sulfasalazine administration
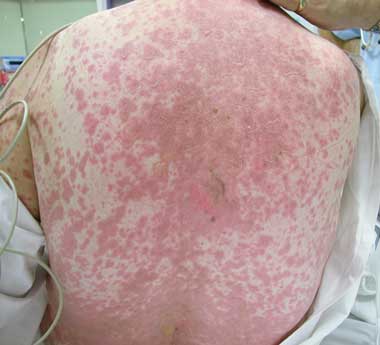
Courtesy of Department of Dermatology, Alfred Hospital, Melbourne.
7 Evidence-based practice tips*
Drug desensitisation can allow safe administration of the drug in immediate hypersensitivity reactions, even in cases of anaphylaxis (Level III-3).
If a patient taking an angiotensin-converting enzyme (ACE) inhibitor develops angioedema, the cause must be assumed to be the ACE inhibitor until proven otherwise (Level IV).
* Based on National Health and Medical Research Council levels of evidence.22
- Francis C K Thien1
- Department of Allergy Immunology and Respiratory Medicine, The Alfred Hospital, Melbourne, VIC.
The research for this article was supported, in part, by a grant from the Harold Mitchell Foundation.
I am on advisory boards for GlaxoSmithKline and Reckitt Benckiser, for which I receive honoraria. I have received speaker fees from GlaxoSmithKline, Schering-Plough, AstraZeneca and Boehringer-Ingelheim, and travel assistance to attend international meetings from GlaxoSmithKline and AstraZeneca.
- 1. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255-1259.
- 2. Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions. In: Davies DM, editor. Textbook of adverse drug reactions. Oxford: Oxford University Press, 1977: 10.
- 3. Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use. Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113: 832-836.
- 4. Greenberger PA. 8. Drug allergy. J Allergy Clin Immunol 2006; 117 (2 Suppl Mini-Primer): S464-S470.
- 5. Royal Australian College of General Practitioners, Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists, Pharmaceutical Society of Australia. Australian medicines handbook. Adelaide: Australian Medicines Handbook Pty Ltd, 2003.
- 6. MIMS annual. Sydney: CMPMedica, 2006.
- 7. Levine BB. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med 1966; 275: 1115-1125.
- 8. Macy E, Mangat R, Burchette RJ. Penicillin skin testing in advance of need: multiyear follow-up in 568 test result-negative subjects exposed to oral penicillins. J Allergy Clin Immunol 2003; 111: 1111-1115.
- 9. Torres MJ, Romano A, Mayorga C, et al. Diagnostic evaluation of a large group of patients with immediate allergy to penicillins: the role of skin testing. Allergy 2001; 56: 850-856.
- 10. Torres MJ, Blanca M, Fernandez J, et al; ENDA; EAACI Interest Group on Drug Hypersensitivity. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy 2003; 58: 961-972.
- 11. Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy 2004; 59: 1153-1160.
- 12. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004; 59: 809-820.
- 13. Aberer W, Bircher A, Romano A, et al; European Network for Drug Allergy (ENDA); EAACI Interest Group on Drug Hypersensitivity. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy 2003; 58: 854-863.
- 14. Gruchalla RS. 10. Drug allergy. J Allergy Clin Immunol 2003; 111 (2 Suppl): S548-S559.
- 15. Gruchalla RS, Pirmohamed M. Clinical practice. Antibiotic allergy. N Engl J Med 2006; 354: 601-609.
- 16. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis — temporary or permanent? Scand J Infect Dis 1972; 4: 229-230.
- 17. Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med 2001; 345: 804-809.
- 18. Szczeklik A, Nizankowska E, Sanak M, Swierczynska M. Aspirin-induced rhinitis and asthma. Curr Opin Allergy Clin Immunol 2001; 1: 27-33.
- 19. Stevenson DD, Sanchez-Borges M, Szczeklik A. Classification of allergic and pseudoallergic reactions to drugs that inhibit cyclooxygenase enzymes. Ann Allergy Asthma Immunol 2001; 87: 177-180.
- 20. Messerli FH, Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet 2000; 356: 608-609.
- 21. Nussberger J, Cugno M, Amstutz C, et al. Plasma bradykinin in angio-oedema. Lancet 1998; 351: 1693-1697.
- 22. National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Canberra: NHMRC, 2000. http://www.nhmrc.gov.au/publications/_files/cp69.pdf (accessed Aug 2006).
- 23. Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med 2003; 349: 1628-1635.






Abstract
Most drug reactions are pharmacological reactions rather than hypersensitivity reactions.
In assessing drug reactions, a detailed clinical history and careful documentation of reactions are most important.
Elucidating the nature and time course (first versus subsequent exposure, immediate versus non-immediate) of a reaction can help to distinguish immune from non-immune hypersensitivity, as well as IgE-mediated from T cell-mediated allergy.
Skin testing and in-vitro tests are of predictive value for only a limited group of IgE-mediated drug allergic reactions.
Drug provocation challenges can be used to eliminate suspicion of a low-probability drug reaction, find a safe alternative to a proven or probable drug reaction, or as a means of desensitisation.
If a patient taking an angiotensin-converting enzyme (ACE) inhibitor develops angioedema, the cause must be assumed to be the ACE inhibitor until proven otherwise.