Clinical record
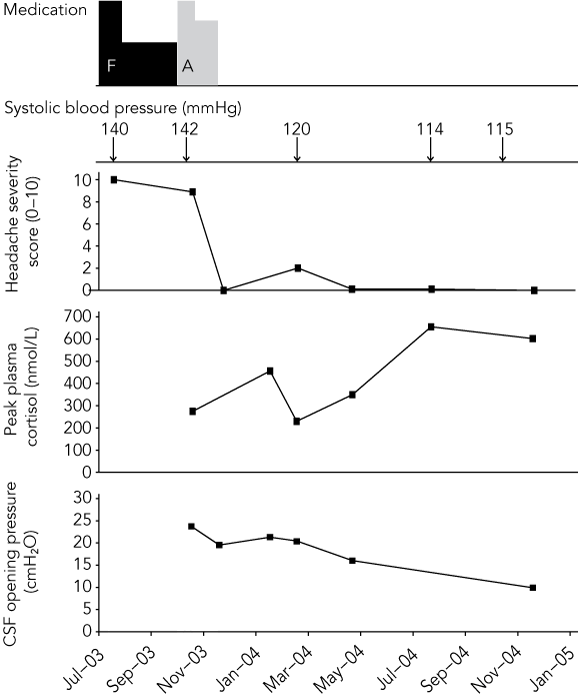
Ophthalmological review now identified a cotton-wool spot on the inferotemporal edge of the right optic disc, suggestive of a past axoplasmic blood flow impediment caused by raised intracranial pressure. The temporal association between his headache severity, CSF opening pressures and cortisol levels is shown in the Box.
Benign intracranial hypertension has an incidence of 1 per 100 000 children.3 As “benign” belies the potentially debilitating nature of this disorder, including the risk of irreversible blindness,4 the synonyms “pseudotumor cerebri” and “idiopathic intracranial hypertension” are sometimes preferred. Although the pathophysiological basis has not been determined, benign intracranial hypertension has been associated with vitamin deficiencies and toxicities, obesity, head injury,5 adrenal insufficiency,6 and medications, including topical and systemic steroids.7-9 To the best of our knowledge, this is the first reported association between benign intracranial hypertension and inhaled corticosteroids (we conducted a MEDLINE search, using multiple search terms, of English language publications from 1966 to June 2005).
The diagnosis of benign intracranial hypertension in our case was based on modified Dandy’s criteria, an elevated cerebrospinal fluid (CSF) opening pressure (using the paediatric reference range),1,2 and repeated instances of immediate headache relief with lumbar puncture. The ophthalmological findings were non-specific but supported the diagnosis. Magnetic resonance venography would have excluded venous sinus thrombosis (an important cause of benign intracranial hypertension), but there was no reason to suspect a hypercoagulable tendency.
Given the absence of papilloedema at presentation, and the fact that the headache and elevated CSF opening pressure were clearly temporally associated with the withdrawal of inhaled corticosteroids, we suggest that the benign intracranial hypertension was related to the withdrawal of the steroid dose. Benign intracranial hypertension has previously only been reported in cases of primary adrenal insufficiency (Addison disease),6 or in association with the use or withdrawal of topical, oral or intranasal corticosteroids.7-9
Clinicians should be mindful of benign intracranial hypertension as a potential side effect when prescribing inhaled corticosteroids. Our case emphasises the importance of using appropriate inhaled corticosteroid dosage and “back titration”. Patients receiving inhaled corticosteroids require regular review. Dosages should be monitored to achieve the minimal dose for symptom control.10
- 1. Soler D, Cox T, Bullock P, et al. Diagnosis and management of benign intracranial hypertension. Arch Dis Child 1998; 78: 89-94.
- 2. Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology 2002; 58: 1551-1553.
- 3. Gordon K. Pediatric pseudotumor cerebri: descriptive epidemiology. Can J Neurol Sci 1997; 24: 219-221.
- 4. Wall M. Idiopathic intracranial hypertension. Semin Ophthalmol 1995; 10: 251-259.
- 5. Dhiravibulya K, Ouvrier R, Johnston I, et al. Benign intracranial hypertension in childhood: a review of 23 patients. J Paediatr Child Health 1991; 27: 304-307.
- 6. Condulis N, Germain G, Charest N, et al. Pseudotumor cerebri: a presenting manifestation of Addison’s disease. Clin Pediatr (Phila) 1997; 36: 711-713.
- 7. Grant DN. Benign intracranial hypertension: a review of 79 cases in infancy and childhood. Arch Dis Child 1971; 46: 651-655.
- 8. Bond DW, Charlton CP, Gregson RM. Drug points: benign intracranial hypertension secondary to nasal fluticasone propionate. BMJ 2001; 322: 897.
- 9. Neville BGR, Wilson J. Benign intracranial hypertension following corticosteroid withdrawal in childhood. BMJ 1970; 3: 554-556.
- 10. van Asperen PP, Mellis CM, Sly PD. The Thoracic Society of Australia and New Zealand. The role of corticosteroids in the management of childhood asthma. Med J Aust 2002; 176: 168-173. <MJA full text>





None identified.