Clinical record
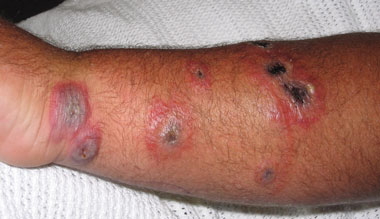
Two days after admission, a nurse at the hospital noted the appearance of the lesions and discussed the patient with infectious disease control officers in the population health unit. The nurse provided photographs, and the characteristic appearance of the lesions in a farmer from the anthrax belt raised the suspicion of cutaneous anthrax. (Figure) The centres of the lesions were depressed and some had formed black scabs. The surrounding tissue was red and oedematous with extensive swelling. These were noted as characteristic features of cutaneous anthrax eschars.1 The local laboratory then performed a gram stain of the Bacillus sp. This suggested Bacillus anthracis, and the isolate was forwarded the following day to the Institute of Clinical Pathology and Medical Research.
Reference laboratory investigation
The B. anthracis-specific polymerase chain reaction (PCR) assay was performed.2 Briefly, this is a PCR system that detects five target gene sequences present in the chromosome, and virulence plasmids pX01 and pX02. The chromosomal marker (Ba813) is present in all B. anthracis strains. Virulent strains of B. anthracis are encapsulated and toxigenic. Plasmids pX01 and pX02 are both required for virulence, and the absence of either results in attenuation. The PCR assay on this organism gave a positive result for all five targets
Anthrax is a bacterial infection resulting from endospores of Bacillus anthracis, a gram-positive, rod-shaped bacterium, entering the body through skin abrasions or by inhalation or ingestion.1 The cutaneous form accounts for more than 90% of all human cases of anthrax worldwide.3 Anthrax is a zoonosis, and normally affects grazing animals such as sheep, cattle and goats. Animals usually become infected by ingesting anthrax spores, which remain viable in the soil for many years; the spores are resistant to desiccation and ultraviolet light.4
In New South Wales, anthrax in animals is a notifiable disease under the Stock Diseases Act 1923 administered by the NSW Department of Primary Industries (DPI). The DPI has responsibility for monitoring known anthrax properties and controlling outbreaks of animal anthrax. Most outbreaks in NSW occur in the “anthrax belt”, which runs through the middle of NSW and into northern Victoria. The area is bordered by Moree and Bourke in the north and Albury and Deniliquin in the south. About three properties per year in NSW experience an anthrax outbreak.5
Cutaneous anthrax in humans usually results from direct contact with infected animals or animal products such as wool, meat or hides, and is generally an occupational hazard. The head, forearms and hands are the most common sites of infection.4 The lesions are not usually painful, but pain may result from oedema or secondary infection. The differential diagnosis includes conditions producing papular lesions with regional lymphadenopathy. If the lesions are purulent, staphylococcal lymphadenitis is the most likely cause, although secondary staphylococcal infection may occur with cutaneous anthrax.1
Human cutaneous anthrax is rare in Australia, with the most recent cases reported in 1998 in a forklift driver in Queensland and in 1997 in a knackery worker in northern Victoria.6 The last known case in NSW occurred in 1991, in a worker who was slaughtering sheep on a property in the anthrax belt.7
Lessons from practice
Human cutaneous anthrax may occur in Australia, associated with occupational exposure to infected stock or products contaminated with anthrax spores.
Cutaneous anthrax eschars characteristically appear as depressed ulcers with black scabs surrounded by oedema, and are painless and not purulent.
Secondary infection may produce purulent lesions and mask the clinical and laboratory diagnosis of cutaneous anthrax.
Health care workers and laboratory staff in the anthrax belt should be aware of the clinical and laboratory aspects of cutaneous anthrax.
The source of infection in our patient remains unclear, despite the farm being an anthrax-prone property. The farmer had not reported any recent animal anthrax on the property, although animal outbreaks had occurred in previous years. The spores might have been introduced directly from the soil at the time of the injury, or entered the wounds later following contact with soil, animals or other products contaminated with anthrax spores. Animal anthrax is more likely to occur following a climate change such as heavy rain after a prolonged drought.5,8 About a week before the farmer sustained the injury, the property, which had experienced drought for number of years, received more than 40 mm of rain.
Public health action included discussion of the nature of the infection with the patient and staff of the health facility, who were reassured that cutaneous anthrax is not transmissible from person to person and that the vegetative form of the bacterium is not infectious.1 Contact was made with the regional veterinary officer of the DPI, according to arrangements that provide for the exchange of information on zoonotic diseases.
Routine vaccination and surveillance of stock, as well as the rapid identification and management of animal anthrax incidents are key actions required to reduce human cutaneous anthrax. Additionally, a high level of awareness among farm workers and health care staff will support the early diagnosis and appropriate treatment of human cases. Prevention through vaccination is not possible in Australia, as a human anthrax vaccine is not registered in Australia.9
The United States Centers for Disease Control and Prevention (CDC) do not recommend prophylaxis for the prevention of cutaneous anthrax. Active surveillance is recommended where there is a continuing risk of exposure.10 Prophylaxis for inhalation anthrax exposure is recommended as a 6-week course of ciprofloxacin or doxycycline.11 Inhalation anthrax results from breathing in large numbers of anthrax spores. This concentration of spores is not usually reached in soil, and inhalation anthrax has not been described in Australia.8
The characteristic eschar lesions and the absence of fever, pain and pus, along with a history of contact with animals or animal products, should suggest anthrax.1 However, secondary infection, as occurred in our patient, can produce purulent lesions and fever and mask the initial cause of infection. Cutaneous anthrax is generally self-limiting and resolves without complications. The infection responds rapidly to antibiotic treatment, and oral penicillin is generally highly effective for cutaneous disease, rendering lesions sterile after 24 hours.1 The CDC now recommend oral ciprofloxacin or doxycycline for cutaneous anthrax. This recommendation follows the use of spores of a β-lactamase-positive anthrax strain as a bioterrorism agent.12 Spread to regional lymph nodes and septicaemia occurs in about 20% of untreated cases of cutaneous anthrax.3 Intravenous therapy with a multidrug regimen is recommended for cutaneous anthrax with systemic involvement.12
- 1. Dixon TC, Meselson M, Guillemin J, et al. Anthrax. N Engl J Med 1999; 341: 815-826.
- 2. Ramisse V, Patra G, Garrigue H, et al. Identification and characterisation of Bacillus anthracis by multiplex PCR analysis of sequences on plasmids pX01 and pX02 and chromosomal DNA. FEMS Microbiol Lett 1996; 145: 9-16.
- 3. Spencer RC. Bacillus anthracis. J Clin Pathol 2003; 56: 182-187.
- 4. Heymann DL, editor. Control of communicable diseases manual. 18th ed. Washington, DC: American Public Health Association, 2004.
- 5. Anthrax. Primefact 114. NSW Department of Primary Industries, 2006. http://www.dpi.nsw.gov.au/aboutus/resources/factsheets/primefacts/anthrax (accessed Jul 2006).
- 6. Australian Government Department of Health and Ageing. Anthrax fact sheet. Canberra: Department of Health and Ageing, 2003. http://www.health.gov.au/internet/wcms/publishing.nsf/content/health-pubhlth-strateg-communic-factsheets-anthrax_fact.htm (accessed Jul 2006).
- 7. Jalaludin B, Sullivan E. Human cutaneous anthrax — a case report. N S W Public Health Bull 1991; 2: 53. http://www.health.nsw.gov.au/public-health/phb/phbjun91.pdf (accessed Jul 2006).
- 8. Queensland Department of Primary Industries and Fisheries. Anthrax in livestock. Brisbane: Queensland DPIF, 2003. http://www2.dpi.qld.gov.au/health/8242.html (accessed Jul 2006).
- 9. Australian Technical Advisory Group on Immunisation. Australian immunisation handbook. 8th ed. Canberra: National Health and Medical Research Council, 2003. http://www9.health.gov.au/immhandbook/ (accessed Aug 2006).
- 10. Centers for Disease Control and Prevention. Suspected cutaneous anthrax in a laboratory worker — Texas, 2002. MMWR Morb Mortal Wkly Rep 2002; 51: 279-281.
- 11. Centers for Disease Control and Prevention. Update: investigation of anthrax associated with intentional exposure and interim public health guidelines. MMWR Morb Mortal Wkly Rep 2001; 50: 889-897.
- 12. Centers for Disease Control and Prevention. Update: investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy, October 2001. MMWR Morb Mortal Wkly Rep 2001; 50: 909-919





The authors acknowledge Ms Helen Thatcher who took the photograph.
None identified.