The purpose of this supplement is to provide medical and health professionals with a review of the health benefits of herbs and spices.
Summary — Linda C Tapsell
The historical and cultural use of herbs and spices — Ian Hemphill and Lynne Cobiac
Herbs and spices as functional foods — Linda C Tapsell
The health benefits of herbs and spices: how strong is the evidence?
Cardiovascular disease — Craig S Patch and David R Sullivan
Cancer — Michael Fenech
Mental health and cognition — Steven Roodenrys
Type 2 diabetes mellitus — Jennifer B Keogh and Peter M Clifton
Osteoarthritis and inflammatory response — Craig S Patch
Public health — Peter G Williams
Dietary implications — Virginia A Fazio and Karen E Inge
Moving forward — Linda C Tapsell
Professor Linda C Tapsell, PhD, MHPEd, DipNutrDiet, FDAA, Director, National Centre of Excellence in Functional Foods, University of Wollongong, Wollongong, NSW.
Mr Ian Hemphill, Director, Herbie’s Spices, Sydney, NSW.
Dr Lynne Cobiac, PhD, MBA(Advanced), PostGradDipNutrDiet, Business Manager, Preventative Health National Research Flagship, CSIRO, Adelaide, SA.
Associate Professor David R Sullivan, FRACP, FRCPA, FCANZ, Clinical Associate Professor, Department of Clinical Biochemistry, Royal Prince Alfred Hospital, Sydney, NSW.
Dr Michael Fenech, PhD, Principal Research Scientist, CSIRO Human Nutrition, Adelaide, SA.
Dr Craig S Patch, PhD, MBA, GradDipNutrDiet, Industry Projects Manager, National Centre of Excellence in Functional Foods, University of Wollongong, Wollongong, NSW.
Associate Professor Steven Roodenrys, PhD, Associate Professor, School of Psychology, University of Wollongong, Wollongong, NSW.
Ms Jennifer B Keogh, MSc, DipDiet, Research Dietitian, CSIRO Human Nutrition, Adelaide, SA.
Professor Peter M Clifton, PhD, FRACP, Theme Leader Obesity, CSIRO Human Nutrition, Professor of Medicine, University of Adelaide, Adelaide, SA.
Associate Professor Peter G Williams, PhD, MHP, DipNutrDiet, Director, Smart Foods Centre, University of Wollongong, Wollongong, NSW.
Ms Virginia A Fazio, MSc, MBA, GradDipDiet, Senior Consultant Dietitian, Institute of Health and Fitness, Melbourne, VIC.
Ms Karen E Inge, BSc, DipDiet, Director, Institute of Health and Fitness, Melbourne, VIC.
Dr Craig S Patch, PhD, MBA, GradDipNutrDiet, Industry Projects Manager, National Centre of Excellence in Functional Foods, University of Wollongong, Wollongong, NSW.
Dr Craig S Patch, National Centre of Excellence in Functional Foods, University of Wollongong, Wollongong, NSW 2522.
Phone: +61 2 4221 5125; Fax: +61 2 4221 4844
Herbs and spices have a traditional history of use, with strong roles in cultural heritage, and in the appreciation of food and its links to health. Demonstrating the benefits of foods by scientific means remains a challenge, particularly when compared with standards applied for assessing pharmaceutical agents. Pharmaceuticals are small-molecular-weight compounds consumed in a purified and concentrated form. Food is eaten in combinations, in relatively large, unmeasured quantities under highly socialised conditions. The real challenge lies not in proving whether foods, such as herbs and spices, have health benefits, but in defining what these benefits are and developing the methods to expose them by scientific means.
Generally, the leaf of a plant used in cooking may be referred to as a culinary herb, and any other part of the plant, often dried, as a spice. Spices can be the buds (cloves), bark (cinnamon), roots (ginger), berries (peppercorns), aromatic seeds (cumin), and even the stigma of a flower (saffron). Many of the aromatic seeds known as spices are actually gathered from plants when they have finished flowering. A familiar example would be coriander, with the leaves being referred to as a herb, and the dried seeds as a spice. When referring to the stem and roots of coriander, which are used in cooking, and to onions, garlic and the bulb of fennel, these parts of these plants tend to be classified along with herbs, as they are often used fresh and applied in a similar way to cooking.
Herbs and spices have a long history of both culinary use and of providing health benefits, as well as acting as preservatives. Ancient Egyptian papyri from 1555 bce record the use of coriander, fennel, juniper, cumin, garlic and thyme.7 It is reported that the Sumerians were using thyme for its health properties as early as 5000 bce, and the farmers of Mesopotamia were growing garlic as early as 3000 bce. An international trade in spices dates back to 4500–1900 bce, mainly with Ethiopia. The ancient Egyptians worshipped garlic, and garlic cloves were found in the tomb of King Tutankhamen. Other Egyptians had wooden cloves of garlic in their tombs to keep the future meals of the afterlife tasty, wholesome and long-lasting.8 Dried mint leaves have been found in Egyptian pyramids dating around 1000 bce.9 The Egyptians reportedly fed large amounts of radishes, onions and garlic to their slaves, ostensibly to keep them healthy.7 Cardamom and cinnamon (traded from Ethiopia) were also used extensively in ancient Egypt as spices, but less so for medicinal purposes. The Assyrians in Mesopotamia (a country now incorporated by modern day Iraq and Iran) also developed knowledge around the health benefits of herbs, and refer to juniper, saffron, and thyme around this time.7
In ancient Greece and Rome, herbs appear to have been used more than spices. Hippocrates (460–377 bce) had a repertoire of 300 remedies that included garlic, cinnamon and rosemary, all of which were locally available.7 He reportedly used garlic to treat uterine cancer. Mint was highly valued for its positive effects on the digestive system, and liquorice was used as a sweet, but also as a herb for its anti-inflammatory actions and for asthma, chest problems and mouth ulcers. Rosemary was used to improve and strengthen memory — and is sometimes still burnt in the homes of Greek students taking exams. Around the first century ce, Pedanius Dioscorides — Greek physician, botanist, pharmacologist and surgeon — published the first plant monograph that included 600 herbs, describing how to choose, store and apply plants for a range of health benefits. Another Greek physician, Galen (131–200 ce), who lived in Rome from 162 ce, had a strong influence on the development of herbal remedies, but used complicated mixtures, containing up to 100 ingredients.7 Dioscorides’ monograph was used as a principal reference in Europe until the 17th century.9
In China, the use of plants for health benefits is shrouded in legend. Two legendary Chinese emperors are credited with discovering and recording the medicinal properties of herbs — Sheng Nong, the Divine Husbandman (2838–2698 bce), and Huang Di, the Yellow Emperor (2698–2598 bce).7 Traditionally, the Chinese have integrated food, nutrition and health, and will often include herbs and spices in specially prepared soups, dishes or beverages for both sustenance and for purported health benefits. Ginseng and Ginkgo biloba are reportedly used to improve stamina and cognitive performance, respectively. Other examples include the use of galangal for abdominal pain, nutmeg for diarrhoea, and cinnamon for colds and flu.7
In India, the traditional medicine, Ayurveda, evolved more than 5000 years ago in the Himalayas, with knowledge transmitted orally until it was written down in Sanskrit poetry — the Vedas — around 1500 bce. It flourished in the 7th century. Ayurveda focuses on disease prevention and health promotion, with an emphasis on diet.10,11 Examples of Ayurvedic use of herbs and spices for health effects include turmeric for jaundice, basil to protect the heart, mace for stomach infections, cinnamon to stimulate circulation, and ginger as the universal medicine, in particular for relieving nausea and indigestion. Many of these herbs and spices are used in Indian cooking to impart flavour, and significant quantities can be consumed in one meal. It has been reported that such herbs and spices can supply reasonable quantities of nutrients as well, such as iron. It has been estimated that an adult in India can eat as much as 4 g of turmeric daily, which could provide 80–200 mg/day of the bioactive component curcumin. Some Indians have been reported to eat as much as 50 g of garlic in a week.12
With the decline of the Roman Empire around 476 ce, the development of Arabic medicine in 500–1300 ce preserved some of the knowledge surrounding the health benefits of herbs and spices, and built on the knowledge of Galen.7 The spread of Islamic culture into north Africa had profound effects in the region, blending their knowledge with that from China and India.
In the 9th century, the Emperor Charlemagne is quoted as saying, “a herb is a friend of physicians and the praise of cooks”, suggesting that the dual role of herbs and spices for flavouring and for health benefits was still recognised. During the 11th century, the knowledge of Arabic medicine filtered back to Europe, and by the 13th century, trade with Africa and Asia was bringing in new herbs and spices. Around this time, galangal was called the “spice of life”.7 Garlic was used by herbalists during the plague.7 Later, Louis Pasteur (1822–1895 ce) found that it killed bacteria, and it was even used on the battlefields to prevent gangrene.
Mediterranean diets have been associated with reduced incidence of some chronic diseases, such as heart disease and cancer.13 While dietary studies are complex, Mediterranean diets do include considerable amounts of garlic, rosemary, basil and thyme, among other herbs, which may help to explain some of the protective effects observed in populations following more traditional Mediterranean diets.
In Australia, the Indigenous population developed its own local herbal medicine based on the plants that were available. Their isolation meant that the Indigenous population did not encounter Western diseases, and so the use of herbs and plants was developed for less serious disorders. Examples include the use of river mint for coughs and colds, and wattle and eucalyptus for diarrhoea, fever, headache, and a range of other ailments.14
Examining herbs and spices from a functional food perspective might begin with how herbs and spices are used in the diet. There is no single definition of functional foods, but there are many contexts in which the concept is played out, including scientific endeavour, technological advancement, food marketing, and food standards regulation.15 From a scientific perspective, functional foods have been defined as “foods that provide benefit beyond basic nutrition”.16 This definition draws on notions of food (ie, a recognisable unit of consumption in contradistinction to drugs), notions of benefits (which implies the need for scientific evidence), and the notion of “basic nutrition” (a concept open to interpretation). In some ways, basic nutrition might actually reflect the current depth of nutrition knowledge and practice. Thus, meeting requirements for vitamins and minerals (which have recommended reference values)17 could be considered basic nutrition. The underlying view is that these nutrients are required to maintain normal bodily function. However, the way in which food components of today are studied is not limited to concepts of preventing clinical deficiency and maintaining homeostasis, but includes a growing recognition of the way in which food components actively interact with the body to support health and prevent abnormality and overt disease.18
Herbs and spices fit into this picture in a number of ways. In this supplement, the focus is on their role in the diet rather than their use as medicines. Establishing this role would involve identifying unique bioactive compounds to help identify target benefits. Research would then be conducted on the food itself (supplements), a meal based on the food (acute effects), or the food in a whole diet in which the observed benefits can be attributed to the specified combination. The traditional use of foods in various cultures provides many clues to this development. For example, certain meals in traditional Thai cuisine have a cultural history of supporting health based on their combination of herbs, spices and other foods,19 so that it might be better for dietary guidelines to reference dishes, rather than single foods as we do in Western societies (which tend to focus on targeted nutrients being delivered by core food groups). The real challenge then comes from defining benefits and providing the scientific evidence for these benefits.
Craig S Patch and David R Sullivan
Most evidence concerning the cardiovascular effects of culinary herbs and spices relates to the possible impact of garlic and garlic oil. Consumption of garlic or garlic oil has been associated with a reduction in total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride levels. Studies suggest that an intake of between half and one garlic clove per day can reduce cholesterol by 9%.20,21 This finding is consistent with a more recent meta-analysis of 13 placebo-controlled trials involving 781 patients taking garlic supplements. The authors concluded that intake of 600–900 mg of standardised garlic extract per day was associated with a modest 0.41 mmol/L decrease in serum cholesterol level.22 Although this was verified in the most current and comprehensive review,23 the authors highlighted that the six most rigorous trials showed a non-significant trend (Box 1).24-29
It has been suggested that this variation of effect may reflect a loss of active compounds during processing, or an inhibition in the enzymatic release of the active compounds from garlic.25 Allicin has been proposed as the primary active compound, although the mechanism of action is still not well understood. Allicin is not present in fresh garlic and is converted from the precursor alliin within seconds of being crushed or chewed. Allicin is rapidly absorbed in the small intestine and converted to allyl mercaptan and allyl methyl sulfide soon after absorption.30 In addition, factors related to food preparation, manufacturing, and in-vivo metabolism affect the bioactivity of allicin and may explain some of the heterogeneity of results found in these meta-analyses.21,22 With few exceptions, the more recent published trials have used dietary supplemental forms of garlic, rather than garlic as a food.30 This has significant implications, as a study found that the allicin yield of 24 commercial brands of garlic tablets averaged 14% of the amount claimed on the label.21,31 Of the brands used in most of the clinical trials since 1995, the active ingredient was found to be only 2%–18% of that claimed on the label.32
A number of clinical trials have examined the effect of garlic on cardiovascular risk factors other than lipoproteins and lipid levels. Garlic extracts have been associated with anticlotting effects,33 as well as modest reductions in blood pressure (about a 5.5% decrease in systolic pressure).34 However, of the 33 published studies with data on blood pressure, only four included patients with hypertension.6
Data on the effects of other herbs and spices are limited. In one trial, participants with hypercholesterolaemia who consumed 140 mg of lemon grass (Cymbopogon citratus) oil daily experienced a drop in cholesterol concentrations by up to 38 mg/dL, but this trial had no control group.35 Spice components like ginger, capsaicin and curcumin have been associated with a decrease in LDL cholesterol and an increase in high-density lipoprotein cholesterol levels, but these results have been limited to rat studies.36,37
The putative protective heart health benefits of antioxidants such as flavonoids have been extensively studied. A longitudinal study of 805 elderly men found that daily flavonoid intake from fruit, vegetables and tea of 25.9 ± 14.5 mg (mean ± SD) was inversely associated with heart disease mortality.38 Herbs and spices have an important role in dietary flavonoid intake. Chamomile, liquorice, onions, rosemary, sage and thyme have high flavonoid contents, but there is little evidence apart from epidemiological studies to support a direct cardiovascular health benefit from these herbs and spices.
In recent years, a substantial body of evidence has indicated that free radicals contribute to cardiovascular disease.39 Oxidative modification of LDL is hypothesised to play a key role during the development of atherosclerosis. The use of antioxidants from dietary sources, including herbs and spices, is a promising alternative to the use of antioxidant supplements. In general, herbs and spices have high antioxidant concentrations that have the potential to inhibit the oxidation of LDL.40,41 Like fruits and vegetables, herbs and spices contain many different classes of antioxidants in varying amounts. It has been shown that the intake of herbs can contribute significantly to the total intake of plant antioxidants.42 A study found that the total phenolic content of culinary herbs ranged from 0.26 mg to 17.51 mg of gallic acid per gram fresh weight (Box 2).43 These values were also found to be higher than traditional medicinal herbs.25 At this stage, evidence of benefit from any form of antioxidant intake is restricted to surrogate markers of cardiovascular disease, such as oxidative damage, rather than clinical outcomes.
As yet, there are no data indicating that herbs and spices have an anticarcinogenic effect in humans, but there are several in-vitro studies and rodent in-vivo studies suggesting that certain herbs and spices may have a chemopreventive effect against the early initiating stages of cancer (Box 3).
Herbs may act through several mechanisms to provide protection against cancer. Certain phytochemicals from herbs or herb extracts have been shown to inhibit one or more of the stages of the cancer process (ie, initiation, promotion, growth and metastases).62-65 Inhibition of phase I (procarcinogen activation) and induction of phase II (carcinogen deactivation) metabolic enzymes by herbal products may account for some of the preventive effects against the induction of gene or chromosomal mutations that may initiate cancer.62,63 For example, diallyl sulfide, a compound in garlic, is an efficient inhibitor of the phase I enzyme cytochrome P450 (CYP)3 IIE1 and significantly increases a variety of phase II enzymes, including glutathione S-transferase, quinone reductase and uridine diphosphate-glucuronosyltransferase, which are responsible for the detoxification of carcinogens.63
Herbs may also protect against oxidative stress and inflammation, both of which are risk factors for cancer initiation and promotion as well as other pathological conditions.64-67 An imbalance between the generation of reactive oxygen species (eg, hydroxyl radical and superoxide radical anion) and cellular antioxidant capacity leads to a state of oxidative stress. Herbs and spices contain several natural water-soluble phenolic acids and flavonoids, such as caffeic acid and quercetin, that can scavenge reactive oxygen species, as well as containing lipid-soluble compounds such as tocopherols, carotenoids and sterols that may protect against the generation of genotoxic lipid peroxidation products, such as trans-4-hydroxy-2-nonenal.
Pro-oxidant and pro-inflammatory stimuli induce the mitogen-activated protein and nuclear factor κB inhibitory protein (IκB) kinases that activate nuclear factor κB (NFκB), enabling its translocation into the nucleus where it causes activation of cyclo-oxygenase-2 (COX-2) expression, subsequent prostaglandin production, and excessive stimulation of cell division that can lead to growth of adenomas.64-67 The pro-inflammatory and pro-oxidant effect on increased cell proliferation, combined with oxidative-stress-induced chromosomal instability, increases risk for carcinogenesis (Box 4). The number of herbs with potential anti-inflammatory activity is impressive. Natural anti-inflammatory compounds found in herbs and spices (such as curcumin, gingerol and capsaicin) appear to operate by inhibiting one or more of the steps linking pro-inflammatory stimuli with COX activation, such as the blocking by curcumin of NFκB translocation into the nucleus. It has been shown recently that the natural anti-inflammatory compounds quercetin, curcumin and silymarin were as effective as indomethacin (a non-steroidal anti-inflammatory drug) in inhibiting aberrant crypt foci in the rat.44
Herbs and spices (or their fractions and constituents) with known anticarcinogenic effects in animal models of cancer include turmeric, basil, rosemary, mint and lemon grass, but there are no published reports on potential chemopreventive effects against cancer for other common spices such as thyme, coriander and dill. Turmeric has been widely used as a spice and colouring agent in foods. Recently, turmeric was found to have chemopreventive effects against cancers of the skin, forestomach, liver and colon, and oral cancer in mice.44-46
Oral treatment with basil-leaf extract significantly elevated the activities of cytochrome P450, aryl hydrocarbon hydroxylase, and glutathione S-transferase, all of which are important in the detoxification of carcinogens as well as mutagens. Moreover, basil-leaf extract was effective in inhibiting carcinogen-induced early-stage cancers in the skin and forestomach of mice.47,68 Orientin and vicenin, two water-soluble flavonoids isolated from the leaves of Indian holy basil (Ocimum sanctum), have shown significant protection against radiation-induced lethality and chromosomal aberrations in vivo.48
A methanol extract of the leaves of the plant Rosmarinus officinalis L. (rosemary) and its constituent carnosol (a phenolic diterpene) inhibited 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ornithine decarboxylase activity (a promoter of cell division via polyamine synthesis), TPA-induced inflammation, arachidonic acid-induced inflammation, TPA-induced hyperplasia, and TPA-induced tumour promotion in mouse skin.49 Commercially available ground rosemary powder was shown to inhibit in-vivo binding of 7,12-dimethylbenz[a]anthracene (DMBA) metabolites to mammary cell DNA in rats,50 suggesting that components of rosemary may inhibit breast cancer. In fact, dietary rosemary and carnosol were both shown to inhibit rat mammary carcinogenesis when DMBA was used as the carcinogen.51 Using the C57BL/6J/Min/+ (Min/+) mouse, a model of colonic tumorigenesis, it was found that dietary administration of 0.1% carnosol decreased intestinal tumour multiplicity by 46%, potentially via its ability to enhance E-cadherin-mediated adhesion and suppress β-catenin tyrosine phosphorylation.69 Carnosol has been shown to inhibit the invasion of highly metastatic mouse melanoma B16/F10 cells in vitro.52 Furthermore, it has been shown to have antioxidant activity and suppresses nitric oxide production and iNOS gene expression by inhibiting NFκB activation, which suggests possible mechanisms for its anti-inflammatory and chemopreventive action.53
Geraniol, an acyclic monoterpene alcohol found in lemon grass (Cymbopogon citratus), was shown to inhibit growth and polyamine biosynthesis in human colon cancer cells.54 Citral (3,7-dimethyl-2,6-octadienal), isolated from the methanol extract of lemon grass, was identified as a novel inducer of the phase-2 enzyme glutathione S-transferase.55 Lemon grass extract reduced the number of putatively preneoplastic lesions and the level of oxidative hepatocyte nuclear DNA injury, as assessed in terms of 8-hydroxydeoxyguanosine production in the liver of male Fischer 344 rats.56 Inhibitory effects of lemon grass extract on the formation of azoxymethane-induced DNA adducts and aberrant crypt foci (a preneoplastic lesion) were recently demonstrated in the rat colon.57
Perillyl alcohol, a naturally occurring monoterpene found in lavender, cherries and mint, caused a 22% reduction in tumour incidence and a 58% reduction in tumour multiplicity in a mouse lung tumour bioassay.58 Rats given spearmint water extract (2% weight/volume) as the sole source of drinking fluid before, during, and after 2-week treatment with a colon carcinogen derived from cooked meat, showed significant reductions in colonic aberrant crypt foci compared with rats given water only.59
As a culinary herb, parsley is regularly consumed and parsley-leaf oil is also used extensively for garnishing and seasoning. Myristicin, a major volatile aroma constituent of parsley, showed high activity as an inducer of the phase 2 enzyme glutathione S-transferase in the liver and small intestinal mucosa of strain A/J albino mice,60,61,70 and a 65% inhibition of tumour multiplicity in a rodent lung cancer model.70
3 Summary of evidence for health effects of herbs and spices on cancer
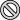 Denotes steps in this carcinogenic process that may be inhibited by certain constituents of herbs and spices.
Denotes steps in this carcinogenic process that may be inhibited by certain constituents of herbs and spices.
There is a very long history in traditional medicine of the use of plants to influence psychological states and processes as well as physical health. In particular, the traditional practices of Ayurvedic medicine in India and Chinese medicine have included treatments for psychological conditions such as anxiety, and preparations to enhance cognitive processes such as memory and attention. Recent decades have seen an increased use of herbal preparations for both of these purposes in Western society despite relatively little scientific research having been conducted to investigate their efficacy.
The herbs that have received the most scientific attention in regard to influencing psychological processes have been drawn from the traditional medicines rather than the culinary herbs. A search of MEDLINE and PsycINFO using the various herb names (eg, basil, coriander) and the terms cognition, memory, attention, dementia and anxiety found only one study of the effect of any of these herbs on psychological processes — it investigated the hypnotic and anxiolytic effects of lemon grass. In this placebo-controlled, double-blind study, lemon grass was taken as a herbal tea for 2 weeks; no effects were found.71
The use of herbal treatments for anxiety is probably the most common example of a herbal influence on mental health. Passiflora incarnata, or passionflower, is approved for use as a sedative by the German Commission E (an expert committee commissioned by the German Government in 1978 to evaluate herbal drugs and preparations from medicinal plants). Valeriana officinalis, or valerian, is probably one of the most widely available herbal treatments, and has been shown to have sedative effects in humans. A review of evidence for behavioural effects and possible chemical pathways for their action concludes that compounds in valerian interact with GABA systems (widespread systems affected by γ-amino butyric acid) in the brain; however, despite identifying flavonoids in passionflower as the likely agent, they do not appear to act on GABA receptors.72
More recently, herbs drawn from traditional Chinese medicine, such as Ginkgo biloba and ginseng, have been advocated for a reputed beneficial effect on cognitive processes.73 There is level I evidence to support the claim that ginkgo can ameliorate cognitive decline in dementia1,74 and level II evidence that it can improve some aspects of memory function in healthy adults.1,75 Further, an extract from ginkgo has been shown to affect cerebral circulation, activity in the cholinergic system, and to have antioxidant properties, all of which may contribute to effects on cognitive function (Box 5).81 However, a population-based study failed to find an effect on the recall of newly learned information after a short delay and the efficiency of working memory processes after a 30-day intervention.75
Consuming herbs can be expected to benefit cognitive function to the extent that they benefit cerebral circulation. For example, it has been shown that people with hypertension perform more poorly on a range of cognitive tasks,82 and cardiovascular disease is associated with impaired cognition.83 Dietary factors that promote cardiovascular health will therefore also maintain cognitive function, although the effect of any single foodstuff is likely to be small.
Perhaps more importantly, antioxidant intake is related to cognitive function because it protects against neuronal degeneration. A review of studies of the effects of antioxidants on Alzheimer’s disease shows quite mixed results; this is attributed to the fact that most studies involve participants who have already been diagnosed with dementia, while epidemiological studies suggest that the most likely benefit of antioxidants is in preventing dementia.76 Some epidemiological studies have shown a relationship between plasma levels of antioxidants and dementia and cognitive impairment.84 At this point in time, the evidence suggests that total antioxidant intake may influence cognitive decline with age through the neuroprotective action of antioxidants.
5 Summary of evidence for health effects of herbs and spices on cognitive function and mental health
Jennifer B Keogh and Peter M Clifton
The biguanide drug metformin, a popular first-line treatment for diabetes, was developed from French lilac (Galega officinalis). To date, this is the only plant-derived treatment for diabetes. At present, the best clinical evidence available for culinary herbs and spices in the treatment of diabetes is for cinnamon, and that is not very convincing. Early in-vitro studies of spices such as cinnamon, cloves, bay leaves and turmeric have shown that they display insulin-enhancing activity.85,86 Mechanistic studies suggest that extracts of cinnamon increase in-vitro glucose uptake and glycogen synthesis and increase insulin-receptor phosphorylation.87-89 A recent study tested the hypothesis that cinnamon would lead to improved glucose tolerance in people with type 2 diabetes mellitus.89 Sixty men and women were randomly assigned to one of six groups. The first three groups were given 1, 3 or 6 g of cinnamon (Cinnamomum cassia extract in capsules) or placebo (same number of capsules). After 40 days, all three doses of cinnamon improved fasting glucose levels (by 18%–29%; P < 0.05).89 However, 20 days later, glucose levels were still lower with the lowest dose only, so it is not clear if any of the effects were due to the spice. In addition, glycated haemoglobin (HbA1c) levels were not measured. It is not clear from this study whether less than 1 g of cinnamon per day would also be beneficial, and this is an important question given that an intake of more than 1 g of cinnamon daily would require supplementation. Triglyceride and LDL cholesterol levels were also lowered by cinnamon, and this again persisted 20 days after cessation of the spice in the two lowest doses, suggesting that a reduction in energy and fat intake had occurred in this group, and this would account for the effects on glucose. A second, double-blind randomised crossover study of 29 postmenopausal women with type 2 diabetes published this year showed that 1.5 g of cinnamon daily had no effect on whole-body insulin sensitivity or oral glucose tolerance, and lipid levels were also unchanged. In conclusion, the effect of cinnamon is still unproven.90
There are many examples of non-culinary herbs that have been tested for their effects on blood glucose, but many of the data are very poor. The 10 most popular plant preparations prescribed by herbalists in Italy for glucose control are gymnema, psyllium, fenugreek, bilberry, garlic, Chinese ginseng, dandelion, burdock, prickly pear cactus and bitter melon.91 Although there is also some evidence for the clinical efficacy of ginseng in managing diabetes, there are concerns about the reproducibility of its effect based on batch, dose and time of taking the herb.92 A 2004 study reported differences in glycaemic effect according to the type of ginseng tested, suggesting that the antihyperglycaemic effect of ginseng can be highly variable.93 A randomised clinical trial in 2000 evaluated ginseng in 19 lean people; 10 without, and 9 with diabetes (Box 6).94 They received 3 g of ginseng or placebo 40 minutes before, or with, 25 g of glucose. Participants (both with and without diabetes) taking ginseng 40 minutes before the glucose showed a significant reduction in glucose level compared with those taking placebo (P < 0.05). There were also reductions in the area under the glycaemic curve for both those with and without diabetes mellitus. In a further study, 10 people without diabetes received, in random order on 12 occasions, 0, 3, 6 or 9 g of ginseng at 40, 80, or 120 minutes before 25 g of glucose. Ginseng reduced postprandial glucose levels (P < 0.05), and 9 g had a slightly greater effect than 3 g.95
Only one study has reported the effect of ginseng ingestion on HbA1c. A 1995 report investigated the effect of ginseng in people newly diagnosed with type 2 diabetes mellitus.96 Over 8 weeks, 36 participants were randomly assigned to receive either ginseng (100 mg or 200 mg) or placebo. Overall, ginseng reduced fasting blood glucose levels and body weight, and the group taking 200 mg of ginseng also showed reduced HbA1c levels. These results suggest that ginseng may have a delaying effect on gastric emptying, although a sulfonylurea-like activity may also play a part.96 In conclusion, evidence supporting the efficacy of non-culinary herbal treatments is limited and, at present, the best evidence for clinical efficacy is for ginseng.
6 Summary of evidence for health effects of herbs and spices on type 2 diabetes
The role that diet plays in minimising the negative effects of ageing has been extensively investigated. Target health issues related to the ageing process include the process of oxidation, the promotion of bone health, memory retention and cognition. With respect to culinary herbs and spices, the best evidence is associated with anti-inflammatory effect, particularly in mediating osteoarthritic pain reduction.
Therapy for osteoarthritis is directed at symptoms, as there is no established disease-altering treatment. Currently, treatment options are confined to pharmacological interventions such as analgesia, anti-inflammatory and intra-articular regimens.98 However, the recent withdrawal of cyclo-oxygenase inhibitors has led many consumers to herbal remedies such as ginger. What follows is a review of the evidence for efficacy of ginger in treating symptomatic osteoarthritis.
Ginger (family Zingiberaceae) is a mixture of over several hundred known constituents, including gingerols, β-carotene, capsaicin, caffeic acid, curcumin and salicylate.99 Animal models have been used for in-vitro and in-vivo testing of formulations and powders of ginger, and have shown that it acts as a duel inhibitor of both cyclo-oxygenase and lipo-oxygenase,100 as well as being an inhibitor of leukotriene synthesis.101 Three trials have tested the effects of ginger extract on pain. A randomised, placebo-controlled crossover study of 46 patients with knee pain showed that ginger had no effect. It was suggested that there may have been a carry-over effect of the ginger extract which blurred the results, as explorative statistics before the crossover suggested a positive effect.102 However, in subsequent studies, ginger extract was found to have a statistically significant effect in reducing knee pain, but the effect was only moderate.103-105 An interesting recent trial of a proprietary combination of iso-α-acids from hops, rosemary extract and oleanolic acid (440 mg/day for 8 weeks) suggested a beneficial effect on pain in patients with arthritis.106 Although these findings seem promising, there is much more to be done in this area. The relative effectiveness of different extracts is unknown, and there are methodological limitations in measuring this. More clinical studies are needed to confirm these results, considering the confounding effects of the total diet.
A few studies, mostly randomised controlled trials, investigating the effect of ginger on osteoarthritis and rheumatoid arthritis have been identified (Box 7).102-104,107,109 Various doses of ginger extract, ranging from 510 mg to 1 g per day, were tested. Pain level was assessed by various validated measures of pain, such as visual analogue scales, Western Ontario and McMaster Universities (WOMAC) osteoarthritis index total score, and SF-12 Health Survey. These trials found that the pain level of the participants in the intervention group was significantly lower than that in the placebo group, and suggests level II evidence1 for the efficacy of ginger in this setting (P < 0.05). In addition, decreased use of non-steroidal anti-inflammatory drugs and analgesics was also observed. However, this improvement was modest103 and the efficacy of ginger treatment was ranked below that of ibuprofen.102
Experimental studies have shown that ginger inhibits the inflammation process. Ginger constituents are duel inhibitors of arachidonic-acid metabolism (a key pathway in inflammation).110 Similar anti-inflammatory activity shown with turmeric (a member of the ginger family) is also suggestive of a potential health benefit.111 In addition, epidemiological studies have indicated that populations that consume foods rich in specific polyphenols (such as ginger) have lower incidences of inflammatory disease.112
7 Summary of evidence for health effects of herbs and spices on arthritis
It is difficult to estimate the current level of consumption of culinary herbs and spices by Australians. The Australian Bureau of Statistics trend data on apparent consumption of foods do not include herbs and spices,113 and results from the last National Nutrition Survey (NNS) in 1995 provide only limited information on consumption — that median daily intake of herbs, spices, seasonings and stock cubes combined was estimated to be 1.4 g per adult (declining with age from 4.2 g in 19–24-year-olds, to 0.7 g in those aged 65 years and older), with only 3.1% of people reported consuming this category of food items on the day of survey.114 The separate intakes of herbs and spices alone are not reported. Intake by males appeared to be higher than that by females, but the low values make it difficult to assess the significance of this difference. In New Zealand, the consumption of spices, estimated using import data, was 364 g per year, or around 1g per day,115 a similar value to that reported in the NNS.
One comparison of spices used in representative vegetable-based and meat-based recipes from 36 different countries found Australia (with a mean of 3.4 spices per recipe) had a moderate level of use compared with the international mean of 3.9 (ranging from 1.6 in Norway to 6.9 in Indonesia).116 However, increased use of herbs and spices as flavourings in foods is a major trend worldwide,117 with sales growth of 20%–30% over the past 5 years in both the United Kingdom and the United States.118 It has been suggested that this trend is partly driven by demographics; as consumers age, their palates can become more adventurous. Promotion can also be important — a recent UK advertisement in which Jamie Oliver encouraged consumers to experiment with nutmeg boosted sales of that spice fourfold.119
Based on retail sales data, consumption of herbs and spices in Australia has increased in line with global trends, and this is expected to continue. The market for local fresh-cut culinary herbs was estimated to be worth over $62 million per year in 2004 and continues to grow at 20% per annum.105 Information from major supermarket sales in 2003 suggests that total retail sales of fresh herbs and spices were valued at $54 million, and sales for dried products were valued at a further $107 million. The sales volumes of fresh herbs are shown in Box 8.
Although there is increasing interest and research in the health-promoting and protective properties of herbs and spices,120-122 there are few authoritative recommendations about intake in existing national dietary guidelines. The first recommendation in the NHMRC dietary guidelines for Australian adults is “Enjoy a wide variety of nutritious foods”,123 and in the NHMRC dietary guidelines for older Australians, a food variety checklist given in an appendix includes the recommendation to use herbs and spices regularly.124 The same food variety checklist has also been used as the basis of a checklist to assess intakes of phytochemical-dense foods, and herbs and spices make up 11 out of the 64 foods in the checklist (basil, oregano, mint, dill/fennel, parsley, pepper, ginger, cumin, turmeric, coriander, rosemary/thyme).125
The two NHMRC dietary guidelines each have another recommendation for using herbs and spices. In the dietary guidelines for adults, the background chapter on choosing foods low in salt states that among the recommended substitutes for salt are ingredients such as curry spices, garlic and onion, and herbs.123 The guidelines for older Australians note particularly that age-related sensory loss of smell and taste is common in older people, especially those who take many medications, and can have adverse effects on overall nutrient intake. The guidelines recommend experimentation with new flavourings such as herbs and spices to stimulate appetite and support adequate overall intakes.124
A few other countries have made similar recommendations about herbs and spices. In the 2005 revision of the Dietary guidelines for Americans, the chapter on choosing a diet moderate in salt and sodium recommends flavouring with herbs and spices.126 Perhaps the country with the most direct recommendation about the health benefits of culinary herbs is Greece. Their dietary guidelines not only refer to the usefulness of herbs as salt substitutes, but also state: “oregano, basil, thyme and other herbs grown in Greece are good sources of antioxidant compounds”.127 This emphasis on the health-promoting properties of herbs is of interest, given research in Australia that has found that first generation Greek migrants have 35% lower mortality from cardiovascular and overall mortality than Australian-born controls, despite high prevalence of risk factors such as obesity, smoking and sedentary lifestyles. It has been suggested that one of the dietary factors contributing to this lower mortality could be their high intake of antioxidant-rich plant foods, including garlic and herbs.128
Despite the generally supportive statements in the dietary guidelines, the quantitative recommendations for intakes of food in the Australian guide to healthy eating do not yet include suggested intakes of herbs and spices.129 Those recommendations aim primarily to ensure adequate intakes of nutrients for which recommended dietary intakes have already been established, and it is probably too early for there to be more definitive recommendations about foods based on their content of other phytochemicals. It should also be borne in mind that there are possible adverse effects of some spices (such as chilli and peppers) if consumed in large quantities,121 although this is unlikely to be a significant risk at normal levels of use. Thus, the apparent increasing consumption of culinary herbs and spices is certainly a welcome trend that is worthy of closer monitoring, and in future, more explicit recommendations about their place in a healthy diet should be included.
Herbs and spices have only recently captured the attention of the scientific community as providing potential health benefits. As a result, there needs to be a significant investment in human clinical trials to substantiate many of the hypothesised health benefits. Nevertheless, this review provides encouragement for further scientific inquiry. The evidence presented in this review suggests that most of the health effects of herbs and spices on cancer, cardiovascular disease, arthritis and mental health protection may be mediated through their potent antioxidant effects, given the range of activity across the group as a whole (Box 9).42 As science uncovers the role of antioxidants in many degenerative diseases associated with ageing, herbs and spices may have a place as an important source of antioxidants in the diet.
While the bioactivity of individual antioxidants may be known, their effects on health may not be as significant as the combination of the whole class of bioactives working through multiple mechanisms of action. This may well be the case in nutrition generally, where, for example, it is now being argued that the health benefits of n-3 fatty acids are attributed to moderate health effects mediated through multiple pathways rather than a single significant mechanism.139 Like the work undertaken with these essential fatty acids, research is required to uncover the mechanisms by which antioxidants deliver health benefits, and then the impact of exogenous antioxidants in this context. A deeper understanding of this role will help to establish recommended intakes, as has been the case for vitamins and minerals. Finally, an understanding of how antioxidant-rich foods (such as herbs and spices) fit within the context of the whole diet, in balance with all other requirements, will enable research at the clinical level to establish the evidence for their putative place in health promotion and disease prevention.
- 1. National Health and Medical Research Council. How to review the evidence: systematic identification and review of the scientific literature. Canberra: NHMRC, 2000.
- 2. Australian guide to healthy eating. Canberra: Australian Government Department of Health and Family Services, 1998.
- 3. Jacobs DR, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr 2003; 78: 508S-513S.
- 4. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 2003; 78: 517S-520S.
- 5. Contor L, Asp NG. Process for the assessment of scientific support for claims on foods (PASSCLAIM) phase two: moving forward. Eur J Nutr 2004; 43 Suppl 2: ii3-ii6.
- 6. Kris-Etherton PM, Hecker KD, Bonanome A, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med 2002; 113 Suppl 9B: 71S-88S.
- 7. Bellamy D, Pfister A. World medicine: plants, patients and people. Oxford: Blackwell Publishers, 1992.
- 8. Block E. Antithrombotic agent of garlic: a lesson from 5000 years of folk medicine. In: Steiner RP, editor. Folk medicine, the art and the science. Washington DC: American Chemical Society, 1986: 125-137.
- 9. Chevallier A. The encyclopedia of medicinal plants. London: Dorling Kindersley, 1996.
- 10. Govindarajan R, Vijayakumar M, Pushpangadan P. Antioxidant approach to disease management and the role of ‘Rasayana’ herbs of Ayurveda. J Ethnopharmacol 2005; 99: 165-178.
- 11. Thatte UM, Dahanukar SA. Ayurveda and contemporary scientific thought. Trends Pharmacol Sci 1986; 7: 247-251.
- 12. Sainani GS, Desai DB, Gorhe NH, et al. Effect of dietary garlic and onion on serum lipid profile in Jain community. Ind J Med Res 1979; 69: 776-780.
- 13. Keys A. Seven countries: a multivariate analysis of death and coronary heart disease. Cambridge, Mass: Harvard University Press, 1980.
- 14. Hostettmann K, Marston A. Australian medical plants. In: Steiner RP, editor. Folk medicine, the art and the science. Washington DC: American Chemical Society, 1985: 215.
- 15. Katan MB, De Roos NM. Promises and problems of functional foods. Crit Rev Food Sci Nutr 2004; 44: 369-377.
- 16. Palou A, Serra F, Pico C. General aspects on the assessment of functional foods in the European Union. Eur J Clin Nutr 2003; 57: S12-S17.
- 17. National Health and Medical Research Council. Nutrient reference values for Australia and New Zealand including recommended dietary intakes. Canberra: NHMRC, 2006.
- 18. Corthésy-Theulaz I, den Dunnen JT, Ferré P, et al. Nutrigenomics: the impact of biomics technology on nutrition research. Ann Nutr Metab 2005; 49: 355-365.
- 19. Kosulwat V, Ganjanasuntorn N, Charoenkiatkul S, et al. The cultural perspective of Thai diet. Proceedings of the 5th International Conference on Dietary Assessment Methods; 2003 Jan 26–29; Chiang Rai, Thailand. 2003.
- 20. Gore JM, Dalen JE. Cardiovascular disease. JAMA 1994; 271: 1660-1661.
- 21. Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol: a meta-analysis. Ann Intern Med 1993; 119: 599-605.
- 22. Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia. A meta-analysis of randomized clinical trials. Ann Intern Med 2000; 133: 420-429.
- 23. Ackermann RT, Mulrow CD, Ramirez G, et al. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med 2001; 161: 813-824.
- 24. Simons LA, Balasubramaniam S, von Konigsmark M, et al. On the effect of garlic on plasma lipids and lipoproteins in mild hypercholesterolaemia. Atherosclerosis 1995; 113: 219-225.
- 25. Isaacsohn JL, Moser M, Stein EA, et al. Garlic powder and plasma lipids and lipoproteins: a multicenter, randomized, placebo-controlled trial. Arch Intern Med 1998; 158: 1189-1194.
- 26. Superko HR, Krauss RM. Garlic powder, effect on plasma lipids, postprandial lipemia, low-density lipoprotein particle size, high-density lipoprotein subclass distribution and lipoprotein(a). J Am Coll Cardiol 2000; 35: 321-326.
- 27. Gardner CD, Chatterjee LM, Carlson JJ. The effect of a garlic preparation on plasma lipid levels in moderately hypercholesterolemic adults. Atherosclerosis 2001; 154: 213-220.
- 28. Berthold HK, Sudhop T, von Bergmann K. Effect of garlic oil preparation on serum lipoproteins and cholesterol metabolism: a randomized, placebo-controlled trial. JAMA 1998; 279: 1900-1902.
- 29. Neil HA, Silagy CA, Lancaster T, et al. Garlic powder in the treatment of moderate hyperlipidaemia: a controlled trial and meta-analysis. J R Coll Physicians Lond 1996; 30: 329-334.
- 30. Gardner CD, Messina M, Lawson LD, et al. Soy, garlic and ginkgo biloba: their potential role in cardiovascular disease prevention and treatment. Curr Atheroscler Rep 2003; 5: 468-475.
- 31. Lawson LD, Wang ZJ. Low allicin release from garlic supplements: a major problem due to the sensitivities of allinase activity. J Agric Food Chem 2001; 49: 2592-2599.
- 32. Lawson LD, Wang ZJ, Papadimitriou D. Allicin release under stimulated gastrointestinal conditions from garlic powder tablets employed in clinical trials on serum cholesterol. Planta Medica 2001; 67: 13-18.
- 33. Steiner M, Khan AH, Holbert D, et al. A double-blind crossover study in moderately hypercholesterolemic men that compared the effect of aged garlic extract and placebo administration on blood lipids. Am J Clin Nutr 1996; 64: 866-870.
- 34. Silagy CA, Neil HA. A meta-analysis of the effect of garlic on blood pressure. J Hypertens 1994; 12: 463-468.
- 35. Elson CE, Underbakke GL, Hanson P, et al. Impact of lemongrass oil, an essential oil, on serum cholesterol. Lipids 1989; 24: 677-679.
- 36. Gujaral S, Bhumra N, Swaroop M. Effect of ginger oleoresin on serum and hepatic cholesterol levels in cholesterol fed rats. Nutr Rep Int 1978; 17: 183.
- 37. Sambaiah K, Satyanarayana MN. Influence of red pepper and capsaicin on body composition and lipogenesis in rats. J Biosci 1982; 4: 425-430.
- 38. Hertog MG, Feskens EJ, Hollman PC, et al. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993; 342: 1007-1011.
- 39. Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001; 54: 176-186.
- 40. Schwarz K, Bertelsen G, Nissen LR, et al. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur Food Res Technol 2001; 212: 319-328.
- 41. Tanabe H, Yoshida M, Tomita N. Comparison of the antioxidant activities of 22 commonly used culinary herbs and spices on the lipid oxidation of pork meat. Anim Sci J 2002; 73: 389-393.
- 42. Dragland S, Senoo H, Wake K, et al. Several culinary and medicinal herbs are important sources of dietary antioxidants. J Nutr 2003; 133: 1286-1290.
- 43. Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 2001; 49: 5165-5170.
- 44. Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis 2005; 26: 1450-1456.
- 45. Chuang SE, Cheng AL, Lin JK, Kuo ML. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem Toxicol 2000; 38: 991-995.
- 46. Chuang SE, Kuo ML, Hsu CH, et al. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis 2000; 21: 331-335.
- 47. Dasgupta T, Rao AR, Yadava PK. Chemomodulatory efficacy of basil leaf (Ocimum basilicum) on drug metabolizing and antioxidant enzymes, and on carcinogen-induced skin and forestomach papillomagenesis. Phytomedicine 2004; 11: 139-151.
- 48. Vrinda B, Uma Devi P. Radiation protection of human lymphocyte chromosomes in vitro by orientin and vicenin. Mutat Res 2001; 498: 39-46.
- 49. Huang MT, Ho CT, Wang ZY, et al. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res 1994; 54: 701-708.
- 50. Amagase H, Sakamoto K, Segal ER, Milner JA. Dietary rosemary suppresses 7,12-dimethylbenz(a)anthracene binding to rat mammary cell DNA. J Nutr 1996; 126: 1475-1480.
- 51. Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett 1996; 104: 43-48.
- 52. Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol 2005; 69: 221-232.
- 53. Lo AH, Liang YC, Lin-Shiau SY, et al. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis 2002; 23: 983-991.
- 54. Carnesecchi S, Schneider Y, Ceraline J, et al. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J Pharmacol Exp Ther 2001; 298: 197-200.
- 55. Nakamura Y, Miyamoto M, Murakami A, et al. A phase II detoxification enzyme inducer from lemongrass: identification of citral and involvement of electrophilic reaction in the enzyme induction. Biochem Biophys Res Commun 2003; 302: 593-600.
- 56. Puatanachokchai R, Kishida H, Denda A, et al. Inhibitory effects of lemon grass (Cymbopogon citratus, Stapf) extract on the early phase of hepatocarcinogenesis after initiation with diethylnitrosamine in male Fischer 344 rats. Cancer Lett 2002; 183: 9-15.
- 57. Suaeyun R, Kinouchi T, Arimochi H, et al. Inhibitory effects of lemon grass (Cymbopogon citratus Stapf) on formation of azoxymethane-induced DNA adducts and aberrant crypt foci in the rat colon. Carcinogenesis 1997; 18: 949-955.
- 58. Lantry LE, Zhang Z, Gao F, et al. Chemopreventive effect of perillyl alcohol on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced tumorigenesis in (C3H/HeJ X A/J)F1 mouse lung. J Cell Biochem Suppl 1997; 27: 20-25.
- 59. Yu TW, Xu M, Dashwood RH. Antimutagenic activity of spearmint. Environ Mol Mutagen 2004; 44: 387-393.
- 60. Zheng G, Kenney PM, Lam LK. Myristicin: a potential cancer chemopreventive agent from parsley leaf oil. J Agric Food Chem 1992; 40: 107-110.
- 61. Ahmad H, Tijerina MT, Tobola AS. Preferential overexpression of a class MU glutathione S-transferase subunit in mouse liver by myristicin. Biochem Biophys Res Commun 1997; 236: 825-828.
- 62. Potter JD, Steinmetz K. Vegetables, fruit and phytoestrogens as preventive agents. IARC Sci Publ 1996; (139): 61-90.
- 63. Wargovich MJ, Woods C, Hollis DM, Zander ME. Herbals, cancer prevention and health. J Nutr 2001; 131 (11 Suppl): 3034S-3036S.
- 64. Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr 2005; 135 (12 Suppl): 2993S-3001S.
- 65. Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol 2002; 40: 1091-1097.
- 66. Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat Res 1998; 402: 259-267.
- 67. Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 1999; 428: 305-327.
- 68. Banerjee S, Prashar R, Kumar A, Rao AR. Modulatory influence of alcoholic extract of Ocimum leaves on carcinogen-metabolizing enzyme activities and reduced glutathione levels in mouse. Nutr Cancer 1996; 25: 205-217.
- 69. Moran AE, Carothers AM, Weyant MJ, et al. Carnosol inhibits β-catenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/+ (Min/+) mouse. Cancer Res 2005; 65: 1097-1104.
- 70. Zheng G, Kenney P, Zhang J, Lam LK. Inhibition of benzo[a]pyrene-induced tumorigenesis by myristicin, a volatile aroma constituent of parsley leaf oil. Carcinogenesis 1992; 13: 1921-1923.
- 71. Leite JR, Seabra Mde L, Maluf E, et al. Pharmacology of lemongrass (Cymbopogon citratus Stapf.). III. Assessment of eventual toxic, hypnotic and anxiolytic effects on humans. J Ethnopharmacol 1986; 17: 75-83.
- 72. Carlini EA. Plants and the central nervous system. Pharmacol Biochem Behav 2003; 75: 501-512.
- 73. Gold PE, Cahill L, Wenk GL. Ginkgo biloba: a cognitive enhancer? Psych Sci Public Interest 2002; 3: 2-11.
- 74. Birks J, Grimley EV, Van Dongen M. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2002; (4): CD003120.
- 75. Stough C, Clarke J, Lloyd J, Nathan PJ. Neuropsychological changes after 30-day Ginkgo biloba administration in healthy participants. Int J Neuropsychopharmacol 2001; 4: 131-134.
- 76. Frank B, Gupta S. A review of antioxidants and Alzheimer’s disease. Ann Clin Psychiatry 2005; 17: 269-286.
- 77. Eidi M, Eidi A, Bahar M. Effects of Salvia officinalis L. (sage) leaves on memory retention and its interaction with the cholinergic system in rats. Nutrition 2006; 22: 321-326.
- 78. Akhondzadeh S, Naghavi H, Vazirian M, et al. Passionflower in the treatment of generalized anxiety: a pilot double-blind randomized controlled trial with oxazepam. J Clin Pharm Ther 2001; 26: 363-367.
- 79. Akhondzadeh S, Noroozian M, Mohammadi M, et al. Salvia officianalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther 2003; 28: 53-59.
- 80. Rahman K. Garlic and aging: new insights into an old remedy. Ageing Res Rev 2003; 2: 39-56.
- 81. Howes MJ, Houghton PJ. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav 2003; 75: 513-527.
- 82. Saxby BK, Harrington F, McKeith IG, et al. Effects of hypertension on attention, memory and executive functions in older adults. Health Psychol 2003; 22: 587-591.
- 83. van Exel E, Gussekloo J, Houx P, et al. Atherosclerosis and cognitive impairment are linked in the elderly. The Leiden 85-plus Study. Atherosclerosis 2002; 165: 353-359.
- 84. Cherubini A, Martin A, Andres-Lacueva C, et al. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging 2005; 26: 987-994.
- 85. Khan AH, Bryden NA, Polansky MM, Anderson RA. Insulin potentiating factor and chromium content of selected foods and spices. Biol Trace Elem Res 1990; 24: 183-188.
- 86. Broadhurst CL, Polansky MM, Anderson RA. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem 2000; 48: 849-852.
- 87. Imparl-Radosevich J, Deas S, Polansky MM, et al. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signalling. Horm Res 1998; 50: 177-182.
- 88. Jarvill-Taylor KJ, Anderson RA, Graves DJ. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J Am Coll Nutr 2001; 20: 327-336.
- 89. Khan A, Safdar M, Ali Khan MM, et al. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003; 26: 3215-3218.
- 90. Vanschoonbeek K, Thomassen BJ, Senden JM, et al. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr 2006; 136: 977-980.
- 91. Cicero AF, Derosa G, Gaddi A. What do herbalists suggest to diabetic patients in order to improve glycemic control? Evaluation of scientific evidence and potential risks. Acta Diabetol 2004; 41: 91-98.
- 92. Vuksan V, Sievenpiper JL. Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. Nutr Metab Cardiovasc Dis 2005; 15: 149-160.
- 93. Sievenpiper JL, Hawkins F, Perez C, et al. A systematic quantitative analysis of the literature of the high variability in ginseng (Panax spp.): should ginseng be trusted in diabetes? Diabetes Care 2004; 27: 839-840.
- 94. Vuksan V, Sievenpiper JL, Koo VY, et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med 2000; 160: 1009-1013.
- 95. Vuksan V, Stavro MP, Sievenpiper JL, et al. American ginseng improves glycemia in individuals with normal glucose tolerance: effect of dose and time escalation. J Am Coll Nutr 2000; 19: 738-744.
- 96. Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care 1995; 18: 1373-1375.
- 97. Vuksan V, Sievenpiper JL, Wong E, et al. American ginseng (Panax quinquefolius L) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am J Clin Nutr 2001; 73: 753-758.
- 98. Lozada CJ, Altman RD. Management of osteoarthritis. In: Koopman WJ, editor. Arthritis and allied conditions: a textbook of rheumatology. Baltimore: Lippincott, Williams & Wilkins; 2001: 2246-2263.
- 99. Schulick P. Ginger: common spice and wonder drug. Brattleboro, Vt: Herbal Free Press, 1996.
- 100. Mustafa T, Srivastava KC, Jensen KB. Drug development report 9. Pharmacology of ginger, Zingiber officinale. J Drug Dev 1993; 6: 25-39.
- 101. Kiuchi F, Iwakami S, Shibuya M, et al. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull (Tokyo) 1992; 40: 387-391.
- 102. Bliddal H, Rosetzsky A, Schlichting P, et al. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthritis Cartilage 2000; 8: 9-12.
- 103. Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum 2001; 44: 2531-2538.
- 104. Wigler I, Grotto I, Caspi D, Yaron M. The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthritis Cartilage 2003; 11: 783-789.
- 105. Parker J. Culinary herbs. In: Salvin S, Bourke M, Byrne T, editors. The new crop industries handbook. RIRDC Publication No. 04/125. Canberra: Rural Industries Research and Development Corporation, 2004: 236-243.
- 106. Lukaczer D, Darland G, Tripp M, et al. A pilot trial evaluating Meta050, a proprietary combination of reduced iso-alpha acids, rosemary extract and oleanolic acid in patients with arthritis and fibromyalgia. Phytother Res 2005; 19: 864-869.
- 107. Sohail M, Chaudhry M, Usman M, et al. Efficacy and tolerance of atrisin in degenerative and inflammatory joint disorders. Phytother Res 2005; 19: 365-368.
- 108. Muhlbauer RC, Lozano A, Palacio S, et al. Common herbs, essential oils, and monoterpenes potently modulate bone metabolism. Bone 2003; 32: 372-380.
- 109. Grzanna R, Lindmark L, Frondoza CG. Ginger — an herbal medicinal product with broad anti-inflammatory actions. J Med Food 2005; 8: 125-132.
- 110. Flynn DL, Rafferty MF, Boctor AM. Inhibition of human neutrophil 5-lipoxygenase activity by gingerdione, shogaol, capsaicin and related pungent compounds. Prostaglandins Leukot Med 1986; 24: 195-198.
- 111. Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa). J Altern Complement Med 2003; 9: 161-168.
- 112. Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol 2005; 16: 77-84.
- 113. Australian Bureau of Statistics. Apparent consumption of foodstuffs, Australia, 1997–98 and 1998–99. Canberra: ABS, 2000. (Cat. No. 4306.0.)
- 114. Australian Bureau of Statistics. National Nutrition Survey: foods eaten, Australia, 1995. Canberra: ABS, 1999. (Cat. No. 4804.0.)
- 115. Fowles J, Mitchell J, McGrath H. Assessment of cancer risk from ethylene oxide residues in spices imported into New Zealand. Food Chem Toxicol 2001; 39: 1055-1062.
- 116. Sherman PW, Hash GA. Why vegetable recipes are not very spicy. Evol Hum Behav 2001; 22: 147-163.
- 117. Sloan AE. The top 10 functional food trends. Food Technol 2005; 59: 20-32.
- 118. Global Information, Inc. Seasonings — UK — August 2005. Mintel International Group Ltd. http://www.the-infoshop.com/study/mt32490-seasonings.html (accessed Jul 2006).
- 119. Sector insight: seasonings — added spice. Marketing 2005; 16: 36.
- 120. Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr 1999; 70 (3 Suppl): 491S-499S.
- 121. Lampe JW. Spicing up a vegetarian diet: chemopreventive effects of phytochemicals. Am J Clin Nutr 2003; 78 (3 Suppl): 579S-583S.
- 122. Billing J, Sherman PW. Antimicrobial functions of spices: why some like it hot. Q Rev Biol 1998; 73: 3-49.
- 123. National Health and Medical Research Council. Food for health — dietary guidelines for Australian adults. Canberra: NHMRC, 2003.
- 124. National Health and Medical Research Council. Dietary guidelines for older Australians. Canberra: NHMRC, 1999.
- 125. Wahlqvist M, Wattanapenpaiboon N, Kannar D, et al. Phytochemical deficiency disorders: inadequate intake of protective foods. Curr Ther 1998; July: 53-60.
- 126. US Department of Health and Human Services; US Department of Agriculture. Dietary guidelines for Americans 2005. Washington DC: US Department of Health and Human Services, 2005. http://www.healthierus.gov/dietaryguidelines/ (accessed Jul 2006).
- 127. Ministry of Health and Welfare Supreme Scientific Health Council. Dietary guidelines for adults in Greece. Arch Hellen Med 1999; 16: 516-524.
- 128. Kouris-Blazos A. Morbidity mortality paradox of 1st generation Greek Australians. Asia Pac J Clin Nutr 2002; 11 Suppl 3: S569-S575.
- 129. Smith A, Kellett E, Schmerlaib Y; Children’s Health Development Foundation; Deakin University. The Australian guide to healthy eating. Background information for nutrition educators. Canberra: Australian Government Department of Health and Family Services, 1998. http://www.health.gov.au/internet/wcms/publishing.nsf/Content/health-pubhlth-publicat-document-fdeduc-cnt.htm (accessed Jul 2006).
- 130. National Public Health Partnership. Eat well Australia: an agenda for action for public health nutrition, 2000–2010. http://www.nphp.gov.au/publications/ (accessed Jul 2006).
- 131. Australian Government, State and Territory health initiative. Go for 2&5 [website]. http://www.gofor2and5.com.au/index.asp (accessed Feb 2006).
- 132. Produce for Better Health Foundation. 5 a day the color way [website]. http://www.5aday.org (accessed Feb 2006).
- 133. WHO Study Group. Diet, nutrition and the prevention of chronic diseases. Geneva: World Health Organization, 1990.
- 134. Stewart H, Harris JM, Guthrie J. What determines the variety of a household’s vegetable purchases? Agric Info Bull 2004; No. 792-3: 1-4. http://www.ers.usda.gov/publications/aib792/aib792-3/aib792-3.pdf (accessed Aug 2006).
- 135. Australian Institute of Health and Welfare. Low fruit and vegetable consumption. Percentage of people with a low intake of fruit and vegetables by age, 2001. http://www.aihw.gov.au/riskfactors/statistics/RFtable2.cfm (accessed Feb 2006).
- 136. Fuhrman B, Volkova N, Rosenblat M, Aviram M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid Redox Signal 2000; 2: 491-506.
- 137. Ninfali P, Mea G, Giorgini S, et al. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br J Nutr 2005; 93: 257-266.
- 138. Samman S, Sandström B, Toft MB, et al. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr 2001; 73: 607-612.
- 139. Harrison N, Abhyankar B. The mechanism of action of omega-3 fatty acids in secondary prevention post-myocardial infarction. Curr Med Res Opin 2005; 21: 95-100.





Gourmet Garden (manufacturer of fresh herb products) has provided finance for the cost of the review and honoraria ($800 per section) to the contributing authors. Gourmet Garden has paid the University of Wollongong, partner in the National Centre of Excellence in Functional Foods, a consultancy fee to develop further materials including consumer education that may reference this publication. Ian Hemphill owns Herbie’s Spices, which is a retail outlet selling herbs and spices in Sydney, mainly to the food service industry. He is a recognised expert in this area and has written books on the subject. Peter Clifton recently completed a consultancy with Gourmet Garden, analysing the antioxidant content of its major herbs. Virginia Fazio and Karen Inge consult to Gourmet Garden on the communication of the application of herbs and spices. Gourmet Garden did not influence the authorship nor comment on any draft of the manuscript.