Off-label (unlabelled or unapproved) prescribing refers to prescribing a registered medicine for a use that is not included or is disclaimed in the product information.1 Examples include use in a different indication, patient age range, dose or route to that which is approved by regulatory authorities. An unlicensed or unregistered medicine is a medicine or dosage form of a medicine that has not been evaluated nor approved in Australia and hence not entered on the Australian Register of Therapeutic Goods.
Much has been published about the extent of, and problems associated with, the off-label and unlicensed use of medicines, particularly in children.2-8 The extent of off-label prescribing is reported to be between 7.5% and 40% in adults,2-5 and may be up to 90% in some hospitalised paediatric patients.6-10 Although the product information may be the main reference for many prescribers, in some cases the best available evidence may not be reflected in the product information,11 so off-label and yet evidence-based prescribing may be the more appropriate choice. This may be because new evidence has become available after marketing (as there is currently no provision for regular updating of the product information) or because there is insufficient incentive for the sponsor to seek an extension of labelling. However, in most cases, adequate research evidence to support off-label prescribing is lacking. A recent survey of 150 million off-label prescriptions in the United States found that 73% had little or no scientific support, even when sources other than the product information were searched.12 Thus, only a small proportion of off-label prescribing may be justified by scientific evidence.
While off-label prescribing is not illegal,13-16 and may sometimes be clinically appropriate (eg, exceptional use in an appropriately informed patient with serious disease, when there are no alternatives and potential benefits outweigh potential risks),17 it brings with it a number of clinical, safety and ethical issues.13,15,18 For example, prescribers may expose children to ineffective therapies and to unknown risks of adverse events by extrapolating from adult data.16 There is now accumulating evidence of resulting harm, with increased incidence and seriousness of adverse drug reactions associated with off-label and unlicensed medicines use in children.19 Furthermore, some long established off-label uses have been shown to either be ineffective or harmful when subjected to proper evaluation (eg, deaths associated with propofol used for sedation in paediatric intensive care).20,21
In contrast to the considerable literature about the extent and consequences of off-label prescribing, there has been no specific guidance to help clinicians trying to make decisions about the appropriateness of such prescribing. Most clinicians perceive off-label prescribing as appropriate and believe that the benefits outweigh the risks.22 However, their awareness of consequences appears to be minimal, with a low level of concern about risk of side effects, unevaluated efficacy and issues surrounding informed consent.23 This raises questions about the validity of their risk–benefit analysis when making decisions about off-label prescribing. Recent legislative and regulatory initiatives in the US20,21 and, more recently, the European Union,24 have provided incentives and inducements for the pharmaceutical industry to undertake more medicines research in children. Eventually these initiatives may reduce the need to consider off-label prescribing in many instances.20 Until such time, practitioners are expected to “use their professional judgement” to determine the appropriateness of off-label use in individual patients,17 although no explicit guidance in exercising such judgement is available. In addition, the legal and ethical ramifications of such prescribing appear to be a source of confusion, with variability in opinions and practice among prescribers and professional organisations.10,14,17
Quality use of medicines (QUM) is defined by the Pharmaceutical Health and Rational use of Medicines Committee as selecting management options wisely; choosing suitable medicines if a medicine is considered necessary; and using medicines safely and effectively. New South Wales Therapeutic Advisory Group (NSW TAG) is an independent state government-funded organisation that aims to promote QUM in the state’s public hospitals and the wider community through collaboration and consensus. The NSW state public health system provides access to nearly 17 000 beds and is responsible for over 1.5 million admissions annually.25 NSW TAG’s membership comprises clinical pharmacologists, directors of pharmacy and other clinicians, representing DTCs from 50 teaching and non-teaching hospitals.
A working party of NSW TAG (Box 1) was established by identifying areas of expertise considered relevant to address the issue of off-label prescribing and to develop recommendations to guide appropriate practice. Nominations were called from NSW TAG members with expertise or special interest in this area. Gaps in expertise (eg, law and ethics) were identified, and appropriate non-TAG members were invited to join the working party. The final working party included hospital-based doctors and pharmacists with expertise in paediatrics, general medicine, oncology, clinical pharmacology and therapeutics, clinical epidemiology, and evidence-based medicine; a consultant in health ethics and law; a representative from the NSW Department of Health; and a representative from the NSW TAG secretariat. The Chair of the working party was a former member of the Australian Drug Evaluation Committee, and two members of the working party are current members of the Pharmaceutical Benefits Advisory Committee.
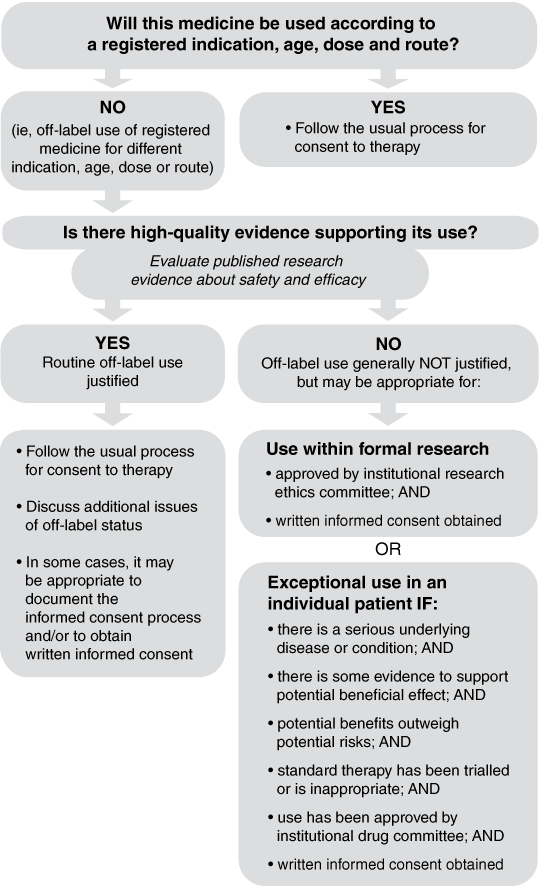
To provide a systematic process for evaluating the appropriateness of any proposed off-label use, a decision algorithm (Box 2) with accompanying explanatory notes was developed. The notes guide clinicians considering off-label use of a particular medicine in answering the question: “Is there high-quality evidence supporting its use?”
In general, the answer to this question is derived from a critical evaluation of the best available patient-based research evidence on both efficacy and safety. The level of rigour of this evaluation should be similar to that used by the Therapeutic Goods Administration for clinical evaluation of medicines submitted for registration approval. Accepted guidelines for critical appraisal of therapeutic studies,26-29 for grading of “strength of evidence”30,31 and for deciding about applicability of research evidence to individual patient circumstances32,33 can be used in answering this question. Various Australian bodies, such as state-based therapeutics advisory groups (eg, NSW TAG) or the National Prescribing Service Ltd, are available to help clinicians (in hospital and community practice) and policymakers (eg, hospital DTCs) evaluate the published literature for new medicines or proposed new uses of existing medicines. Special consideration may need to be given to the needs of special populations (eg, paediatric patients). Systematically developed, evidence-based drug information resources such as the Australian medicines handbook, Therapeutic guidelines, or international resources such as guidance from the National Institute for Health and Clinical Excellence in the United Kingdom or the Canadian Agency for Drugs and Technologies in Health should also be consulted. However, these resources are generally not timely enough to provide useful guidance for very newly marketed medicines,34 which is the category where many off-label uses initially occur.35
Routine off-label use can be justified if there is high-quality evidence supporting efficacy or effectiveness, and sufficient evidence about the medicine’s safety profile to suggest an overall reasonable benefit–risk ratio for a given clinical context (eg, need for longer-term outcome data if a medicine is intended for long-term use). This is especially pertinent with newly marketed medicines, as the available evidence about safety at the time of registration is limited, with a more complete profile emerging only after larger numbers of people have been exposed over a longer period of time. The available efficacy and safety data should be weighed against the seriousness of the underlying condition. As a general rule, the less serious the clinical need, the higher the level of evidence needed to support off-label use of the medicine. Individual patient values and preferences should also be considered.36
Existing guidelines and systems for ranking evidence are focused mostly on efficacy evaluation. The types of studies that should be sought to evaluate the full spectrum of safety of a particular medicine are broader. In many instances, only observational studies (eg, cohort or case–control studies) from post-marketing surveillance, rather than randomised controlled trials or meta-analyses, will provide the necessary data. This applies particularly to rare but potentially serious adverse effects (eg, serious sepsis and death associated with anti-tumour necrosis factor therapy37) or those which manifest after prolonged exposure (eg, hepatotoxicity with low-dose weekly methotrexate for rheumatoid arthritis38) or after a long latent period (eg, infertility after chemotherapy for cancer in childhood39).
In some instances, high-quality research evidence supporting the use of a particular medicine (eg, older off-patent medicines) may not be available and may be unlikely ever to become available. However, there may be extensive experience supporting the efficacy and safety of such medicines. Although such data or “expert opinion” is considered to be of lower quality than high-quality research evidence, there are examples where it may be used to inform decisions about off-label use of a medicine. There are several authoritative medicines compendia that make recommendations for appropriate use supported either by research evidence and/or consensus opinion based on extensive experience with various medicines. These include the Australian medicines handbook, Therapeutic guidelines (Australia) and the British national formulary for children (UK). Other “authoritative” sources may include recommendations from professional societies, although the quality and validity of some of these can be quite variable. Less formal sources of support based on “experience” or “opinion” are less acceptable, and caution is recommended when considering this level of support for off-label use.30,40 It should be emphasised that this category of support for off-label use needs to be systematically reviewed as new research evidence becomes available. For example, some recent US initiatives have stimulated research into off-label uses of medicines in children, generating new evidence about efficacy, safety, and appropriate dosing, with significant implications for long established prescribing practices.20 The current process for determining the content of the product information should be reviewed to allow more timely collation and effective dissemination of available new evidence to all prescribers.
These issues can be addressed by applying the algorithm (Box 2), which identifies three broad categories of appropriate off-label use:
Previous advice from US and UK professional organisations has been that no special provisions for institutional review or informed consent were needed for proposed off-label uses of registered medicines.10,17 However, not all categories of off-label use carry the same level of risk, and so a more explicit approach to the type of patient consent process that might be appropriate is also needed.
Written informed consent: When there is no high-quality evidence supporting routine off-label use of a medicine, there may still be a case for its use in a particular patient (see “exceptional use” criteria in Box 2), but there may be a higher level of risk. In such cases, an independent evaluation of the medicine’s potential benefits and risks should be undertaken (eg, by a DTC) and, if appropriate, approval should be given for individual “exceptional use”. Alternatively, use may occur within the context of a formal research proposal that has been evaluated and approved by an institutional research ethics committee. In either case, written informed consent is required.
A more detailed analysis of the legal and ethical dimensions associated with consent and the administration of off-label medicines can be found at <http://www.ciap.health.nsw.gov.au/nswtag/publications/otherdocs/off_label_use_registered_medicines.pdf>.
These recommendations provide a systematic approach for clinicians and policymakers in evaluating the appropriateness of medicines proposed for off-label use, with a number of resulting benefits. First, by helping to distinguish more explicitly between off-label medicines use supported by scientific evidence and innovative therapy, they should help promote evidence-based prescribing. Second, limiting off-label use of medicines to situations where it is justified by prespecified criteria will reduce inappropriate use (including that which may be inappropriately promoted by the pharmaceutical industry)41 and enhance patient safety by reducing exposure to unnecessary risk. Third, a more explicit process for patient consent will help to better inform patients, parents and carers about the benefits and risks associated with innovative therapy. Fourth, the systematic identification of gaps in knowledge in areas of clinical need will help set the future research agenda, which in turn should result in more useful new knowledge to inform future treatment decisions. Finally, these recommendations may be useful when making decisions about the allocation of scarce health resources.
- Madlen Gazarian1,2
- Maria Kelly3
- John R McPhee4
- Linda V Graudins2,1
- Robyn L Ward1,5
- Terence J Campbell1,5
- 1 University of New South Wales, Sydney, NSW.
- 2 Sydney Children's Hospital, Randwick, Sydney, NSW.
- 3 NSW Therapeutic Advisory Group, Sydney, NSW.
- 4 Centre for Values, Ethics and Law in Medicine, University of Sydney, Sydney, NSW.
- 5 St Vincent’s Hospital, Sydney, NSW.
We acknowledge the valuable contribution to the consensus development process provided by: the Sydney Children’s Hospital Experimental or Un-approved Medication Use Working Party; Dr Debra Kennedy, Director, MotherSafe, (Medications in Pregnancy Advisory Service), the Royal Hospital for Women, Randwick; Ms Karen Kaye, Executive Officer, NSW TAG; NSW Health Pharmaceutical Services Branch.
None identified.
- 1. Turner S. Unregistered and off-label drug use in paediatric inpatients. Aust J Hosp Pharm 1999; 29: 265-268.
- 2. Rayburn W, Farmer K. Off-label prescribing during pregnancy. Obstet Gynecol Clin North Am 1997; 24: 471-479.
- 3. Laetz T, Silberman G. Reimbursement policies constrain the practice of oncology. JAMA 1991; 266: 2996-2999.
- 4. Brosgart C, Mitchell T, Charlebois E, et al. Off-label drug use in human immunodeficiency virus disease. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 12: 56-62.
- 5. Douglas-Hall P, Fuller A, Gill-Banham S. An analysis of off-licence prescribing in psychiatric medicine. Pharm J 2001; 267: 890-891.
- 6. McIntyre J, Conroy S, Avery A, et al. Unlicensed and off label prescribing of drugs in general practice. Arch Dis Child 2000; 83: 498-501.
- 7. Pandolfini C, Impicciatore P, Provasi D, et al. Off-label use of drugs in Italy: a prospective, observational and multicentre study. Acta Paediatr 2002; 91: 339-347.
- 8. Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off-label drug use in paediatric wards in European countries. BMJ 2000; 320: 79-82.
- 9. Cuzzolin L, Zaccaron A, Fanos V. Unlicensed and off-label uses of drugs in paediatrics: a review of the literature. Fundam Clin Pharmacol 2003; 17: 125-131.
- 10. Stephenson T. Medicines for children — the last century and the next. Arch Dis Child 2001; 85: 177-179.
- 11. Cohen JS. Dose discrepancies between the physician’s desk reference and the medical literature, and their possible role in the high incidence of dose-related adverse drug events. Arch Intern Med 2001; 161: 957-964.
- 12. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med 2006; 166: 1021-1026.
- 13. Blum R. Legal consideration in off-label medication prescribing. Arch Intern Med 2002; 162: 1777-1779.
- 14. Kelly M, Gazarian M, McPhee J. Off-label prescribing [letter]. Aust Prescr 2005; 28: 7.
- 15. Collier J. Paediatric prescribing: using unlicensed drugs and medicines outside their licensed indications. Br J Clin Pharmacol 1999; 48: 5-8.
- 16. Gazarian M. Why are children still therapeutic orphans? [editorial]. Aust Prescr 2003; 26: 122-123.
- 17. American Academy of Pediatrics, Committee on Drugs. Uses of drugs not described in the package insert (off-label uses). Pediatrics 2002; 110: 181-183.
- 18. Neubert A, Dormann H, Weiss J, et al. The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients. Drug Saf 2004; 27: 1059-1067.
- 19. European Medicines Agency (EMEA). Evidence of harm from off label or unlicensed medicines in children, London: EMEA, October 2004 (EMEA/11207/04). http://www.emea.eu.int/pdfs/human/peg/1120704en.pdf (accessed Oct 2006).
- 20. Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labelling. Improving the safety and efficacy of pediatric therapies. JAMA 2003; 290: 905-911.
- 21. Schreiner M. Paediatric clinical trials: redressing the imbalance. Nat Rev Drug Discov 2003; 2: 949-961.
- 22. Tan E, Stewart K, Pearce C, et al. Understanding off-label and unlicensed prescribing: combining qualitative and quantitive methodologies. Clin Exp Pharmacol Physiol 2004; 31 Suppl 1: A84.
- 23. Ekins-Daukes S, Helms P, Taylor M, McLay J. Off-label prescribing to children: attitudes and experience of general practitioners. Br J Clin Pharmacol 2005; 60: 145-149.
- 24. European Parliament. Committee on the Environment, Public Health and Food Safety. Draft recommendation for second reading on the Council common position for adopting a regulation of the European Parliament and of the Council on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. http://www.europarl.europa.eu/meetdocs/2004_2009/documents/pr/605/605939/605939en.pdf (accessed Oct 2006).
- 25. NSW Department of Health. Annual report (2002–03). Sydney: NSW Health, 2003: 210.
- 26. Guyatt G, Sackett D, Cook D. How to use an article about therapy or prevention. A. Are the results of the study valid? JAMA 1993; 270: 2598-2601.
- 27. Guyatt G, Sackett D, Cook D. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? JAMA 1994; 271: 59-63.
- 28. Levine M, Walter S, Lee H, et al. How to use an article about harm. JAMA 1994; 271: 1615-1619.
- 29. Oxman A, Cook D, Guyatt G. VI. How to use an overview. JAMA 1994; 272: 1367-1371.
- 30. National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Handbook series on preparing clinical practice guidelines. Canberra: NHMRC, 2000.
- 31. Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ 2001; 323: 334-336.
- 32. Dans AL, Dans LF, Guyatt GH, Richardson S. How to decide on the applicability of clinical trial results to your patient. JAMA 1998; 279: 545-549.
- 33. Craig JC, Irwig LM, Stockler MR. Evidence-based medicine: useful tools for decision making. Med J Aust 2001; 174: 248-253. <MJA full text>
- 34. Ferner R, McDowell S. How NICE may be outflanked. BMJ 2006; 332: 1268-1271.
- 35. Schirm E, Tobi H, de Jong-van den Berg LTW. Risk factors for unlicensed and off-label drug use in children outside the hospital. Pediatrics 2003; 111: 291-295.
- 36. McAlister F, Strauss S, Guyatt G, Haynes R. Integrating research evidence with the care of the individual patient. JAMA 2000; 283: 2829-2836.
- 37. Hyrich K, Silman A, Watson K, Symmons D. Anti-tumour necrosis factor therapy in rheumatoid arthritis: an update on safety. Ann Rheum Dis 2004; 63: 1538-1543.
- 38. Weinstein A, Marlowe S, Korn J, Farouhar F. Low-dose methotrexate treatment of rheumatoid arthritis. Am J Med 1985; 79: 331-337.
- 39. Stevens MCG, Mahler H, Parkes S. The health status of adult survivors of cancer in childhood. Eur J Cancer 1998; 34: 694-698.
- 40. Guyatt G, Sinclair J, Cook D, et al. How to use a treatment recommendation. JAMA 1999; 281: 1836-1843.
- 41. Steinman MA, Bero LA, Chren M-M, Landefeld CS. The promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med 2006; 145: 284-293.





Abstract
Off-label prescribing is the prescription of a registered medicine for a use that is not included in the product information. The practice is common, with rates up to 40% in adults and up to 90% in paediatric patients.
Off-label prescribing is not illegal and may sometimes be clinically appropriate, but is associated with a number of clinical, safety and ethical issues. To date, no explicit guidance has been available to help clinicians assess appropriateness in off-label prescribing.
We describe the development of a guide for clinicians, policymakers and funders of health care in evaluating the appropriateness of medicines proposed for off-label use.
Three broad categories of appropriate off-label use are identified:
off-label use justified by high-quality evidence;
use within the context of a formal research proposal; and
exceptional use, justified by individual clinical circumstances.
An appropriate process for informed consent is proposed for each category.
If there is no high-quality evidence supporting off-label use, and the medicine is not suitable for exceptional or research indications, its use is generally not recommended. This will reduce inappropriate use, enhance patient safety by reducing exposure to unnecessary risk, and may stimulate more clinically relevant medicines research.