Clinical record
A 65-year-old beef-cattle farmer with a 9-year history of sarcoidosis presented with a 2-year history of depressed mood with cognitive decline, weight loss and vomiting. Treatment with three antidepressant medications had proved ineffectual. After bronchoscopic diagnosis, his sarcoidosis had been treated for the ensuing 9 years with prednisolone (initially 30 mg/day and slowly reduced to 2 mg/day). At the time of initial diagnosis, three sputum samples had stained positive for acid-fast bacilli, and the patient was placed on conventional antituberculous therapy. This was ceased after 3 months, following negative mycobacterial cultures of sputa and bronchoscopic specimens. His family history was unremarkable.
On examination, the patient appeared cachectic and older than his stated age. He was vague, with slow speech. Bedside cognitive testing revealed severe memory impairment, with moderate impairment in attention, visuo-constructional ability and executive function. The result of a mini-mental state examination (MMSE) was 17/30. Neurological examination revealed ataxia, brisk tendon reflexes, bilateral habituating palmomental reflexes and proximal weakness. There were no other physical abnormalities.
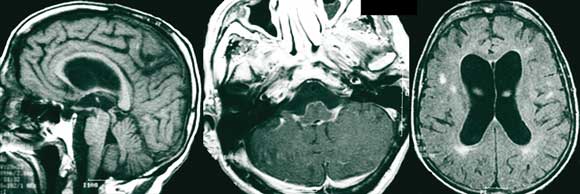
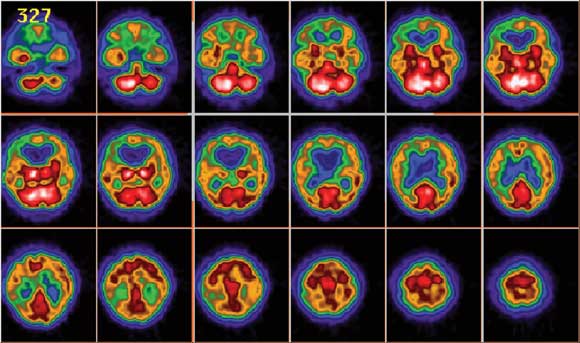
Magnetic resonance imaging (MRI) scans of the brain showed ventricular enlargement, supratentorial white-matter hyperintensities, and extensive leptomeningeal enhancement in the posterior fossa, brain stem and basal cisterns, with extension along the cranial nerves (Figure 1). The MRI results were reported as consistent with neurosarcoidosis. A SPECT (single-photon emission computed tomography) scan, undertaken to differentiate possible causes of dementia, demonstrated global hypoperfusion (Figure 2).
Cerebrospinal fluid (CSF) analysis revealed a raised protein level (3.02 g/L; reference range, 0.15–0.45 g/L) and an opening pressure within the normal range. Cryptococcal antigen was detected in the CSF at a titre of 1 : 4. Although India-ink staining for Cryptococcus was negative, Cryptococcus neoformans var. neoformans was isolated on culture. Serum cultures were negative for fungae. Notably, no malignant cells or bacteria (including acid-fast bacilli) were identified from stained smears. Mycobacterial cultures showed no growth after 8 weeks.
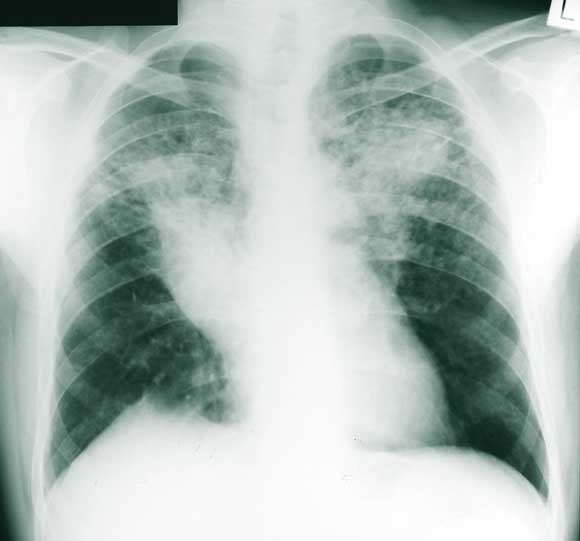
A number of measurements showed that the patient’s sarcoidosis was stable: serum angiotensin-converting enzyme and 24-hour urine calcium levels were normal; respiratory function testing showed moderate air flow obstruction and gas transfer, with no change from previous measurements; and a chest x-ray (Figure 3) showed no new changes.
The patient was diagnosed with cryptococcal meningitis and secondary hydrocephalus. He was initially given antifungal therapy for 4 weeks (intravenous amphotericin B 40 mg a day and oral 5-flucytosine 1.5 g four times a day, followed by intravenous ambisome 200 mg a day when he developed renal impairment with toxic accumulation of 5-flucytosine). Twelve months’ oral fluconazole therapy (400 mg a day) was planned, and a ventriculoperitoneal shunt was inserted. The patient’s mental state had improved when he was re-examined 2 weeks after commencement of therapy (MMSE result, 23/30), and cryptococcal antigen titres fell to levels below detection.
The patient’s longer-term course was complicated by worsening confusion, with chronic subdural haematoma and bilateral posterior cranial fossa infarcts seen on MRI scans done at intervals of several weeks. To ensure that inadequately treated cryptococcosis was not contributing to his cognitive decline, treatment with amphotericin B and 5-flucytosine was reinstituted for 3 weeks. Three months after discharge, the patient was well, with moderate cognitive impairment.
Sarcoidosis is a multisystem granulomatous inflammatory disorder of unknown cause. It affects the lungs in 90% of affected people and the nervous system in 5%–15%. About 5% of people with sarcoidosis have clinically evident neurological involvement (neurosarcoidosis).1 It most commonly presents as a cranial neuropathy, although other presentations — such as aseptic meningitis; mass lesions in the pituitary, hypothalamus, and other brain regions; spinal and peripheral neuropathy; and epilepsy — may occur. Hydrocephalus is uncommon, but can occur secondary to basal obstruction and may present with a progressive dementing syndrome.2 Neuroimaging may reveal focal lesions or periventricular and leptomeningeal enhancement, but results are often non-specific and do not correlate with disease activity.3 Tests on cerebrospinal fluid (CSF) may show an increased white cell count and/or raised protein and angiotensin-converting enzyme levels, but the CSF is normal in up to a third of patients.4
Steroids are the mainstay of treatment for neurosarcoidosis, but recalcitrant illness may require treatment with cytotoxic drugs and irradiation.5 Given the protean nature of presentation of neurosarcoidosis, it is very difficult to diagnose in patients without a history of systemic sarcoidosis. Furthermore, it may be restricted to the central nervous system in some patients.6 Additionally, in patients with known systemic sarcoidosis, the risk is that any new neurological condition will be attributed to the systemic disease without a new diagnosis being sought.2
An association has been postulated between systemic sarcoidosis and infection via inhalation of Cryptococcus neoformans, a dimorphic fungus found in soil. While it is well recognised that cryptococcosis is seen in patients with reduced cell-mediated immunity (such as those with AIDS), patients with sarcoidosis may also have an increased risk of infection independent of immunosuppressive therapy. Up to half of patients with sarcoidosis manifest alterations in cell-mediated immunity.7
The annual incidence of cryptococcal meningitis in Victoria is 4.9 cases per million population, which is lower than the national incidence of 6.6 per million.8 It typically presents with meningism and fever, but may masquerade as Alzheimer’s disease or vascular dementia,9,10 and is often complicated by elevated intracranial pressure or hydrocephalus.11 Typical CSF findings are lymphocytic pleocytosis, elevated protein levels, reduced glucose levels, and, commonly, an increased opening pressure. Cryptococcal meningitis is initially treated with amphotericin B, with or without flucytosine, followed by prolonged oral fluconazole.12 While there are data to support the exclusive use of fluconazole, complicated cases with severe presentations, such as that described in the case presented here, should be treated with amphotericin B and flucytosine.13 Cognitive impairment secondary to communicating hydrocephalus in cases of cryptococcal meningitis often responds to treatment with ventriculoperitoneal shunting.11
The differentiation of cryptococcosis from neurosarcoidosis in a patient with known sarcoidosis is difficult, as there is significant clinical overlap between the two disorders. CSF examination in both may show a raised cell count and protein level, and magnetic resonance imaging (as in our patient) may demonstrate non-specific leptomeningeal enhancement and hydrocephalus. In the setting of suspected neurosarcoidosis in patients with cognitive impairment and clinical or neuroradiological suggestion of meningeal involvement, the exclusion of cryptococcal disease through CSF analysis and culture is mandatory.
Cryptococcal meningitis is among a range of inflammatory, infectious, metabolic and neoplastic/paraneoplastic conditions that can closely mimic the presentation and course of more established dementing disorders, and which can be diagnosed or excluded by a careful combination of clinical assessment and choice of investigations. Although it often presents with symptoms of meningism, it may progress indolently and lead to cognitive impairment (resulting from hydrocephalus) in more than half of individuals.14 It should be considered as a cause of reversible dementia10 in at-risk individuals — including patients with a history of sarcoidosis — and when its presence is suggested by clinical presentation and/or findings on neuroimaging.
- 1. Stern B. Neurological complications of sarcoidosis. Curr Opin Neurol 2004; 17: 311-316.
- 2. Hoitsma E, Faber C, Drent M, Sharma O. Neurosarcoidosis: a clinical dilemma. Lancet Neurol 2004; 3: 397-407.
- 3. Smith J, Matheus M, Castillo M. Imaging manifestations of neurosarcoidosis. AJR Am J Roentgenol 2004; 182: 289-295.
- 4. Nowak D, Widenka D. Neurosarcoidosis: a review of its intracranial manifestation. J Neurol 2001; 248: 363-372.
- 5. Lower E, Broderick J, Brott T, Baughman R. Diagnosis and management of neurological sarcoidosis. Arch Intern Med 1997; 157: 1864-1868.
- 6. Oksanen V. Neurosarcoidosis: clinical presentations and course in 50 patients. Acta Neurol Scand 1986; 73: 283-290.
- 7. Ross J, Katz J. Cryptococcal meningitis and sarcoidosis. Scand J Infect Dis 2004; 34: 937-939.
- 8. Chen S, Sorrell T, Nimmo G, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis 2000; 31: 499-508.
- 9. Aharon-Peretz J, Kliot D, Finkelstein R, et al. Cryptococcal meningitis mimicking vascular dementia. Neurology 2004; 62: 2135.
- 10. Ala T, Doss RC, Sullivan CJ. Reversible dementia: a case of cryptococcal meningitis masquerading as Alzheimer’s disease. J Alzheimers Dis 2004; 6: 503-508.
- 11. Park M, Hospenthal D, Bennett J. Treatment of hydrocephalus secondary to cryptococcal meningitis by use of shunting. Clin Infect Dis 1999; 28: 629-633.
- 12. Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis 2000; 30: 710-718.
- 13. Dismukes WE. Management of cryptococcosis. Clin Infect Dis 1993; 17 (Suppl 2): S507-S512.
- 14. Mamidi A, DeSimone J, Pomerantz R. Central nervous system infections in individuals with HIV-1 infection. J Neurovirol 2002; 8: 158-167.





Damon Eisen receives support from the Clinical Centre for Research Excellence in Infectious Diseases, Victorian Infectious Diseases Service, Royal Melbourne Hospital.
None identified.