Rhesus (Rh) D immunoglobulin (anti-D) is a human blood product provided by a small group of immunised volunteer donors. It has been used since the 1960s in women who are Rh D-negative to prevent Rh D immunisation after giving birth to a baby who is Rh D-positive. Prevention of Rh D immunisation has been a major medical achievement, as Rh D immunisation was a significant cause of perinatal morbidity and mortality in subsequent pregnancies of affected women. In the United Kingdom, for example, the infant mortality from Rh haemolytic disease of the newborn decreased from 46 per 100 000 before 1969 to 1.6 per 100 000 in 1990 as a result of anti-D.1
There are differences internationally in the approach to postpartum anti-D prophylaxis, not only in the dose of anti-D used, but also in whether testing is performed to quantitate the volume of fetomaternal haemorrhage (FMH). In Australia, the UK and the United States, postpartum anti-D doses of 600 IU (120 mg), 500 IU (100 mg) and 1500 IU (300 mg), respectively, are administered to Rh D-negative women. These doses are sufficient to cover Rh D-positive fetal red cell bleeds of 6, 5 and 15 mL, respectively. The FMH volume is then quantitated and additional anti-D is given if necessary.2,3 In other countries (such as Germany and other European countries) a large dose of anti-D (1500 IU) is given, but FMH volume is not quantitated.
In 1999, the National Health and Medical Research Council (NHMRC) issued guidelines on the use of routine antenatal anti-D prophylaxis.4 A phased implementation did not commence until 2002 because of a shortage of domestic supplies of anti-D.2,3,5 As a monoclonal anti-D (enabling production of unlimited quantities) has not yet been developed, maintaining a pool of voluntary immunised donors remains critical to the ongoing supply.6 After an increase in donor recruitment by the Australian Red Cross Blood Service (ARCBS), Australia has now become self-sufficient with respect to anti-D supply, obviating the need to import anti-D.5 One strategy for improving the efficient use of anti-D is to assess whether the amount that is administered routinely postpartum can be reduced. In this article, we report a large retrospective audit of our FMH data, obtained by flow cytometry, to provide support for a dose-reduction strategy.
Within 2 hours of infant delivery, maternal venous blood was collected into EDTA-anticoagulant and transported at 4oC by air or road to the central testing facility in the capital city, Perth, for FMH volume quantitation. All women were given a standard postpartum dose of 625 IU (125 mg) anti-D before the result of the FMH volume quantitation was known, in accordance with Australian guidelines at the time.3
FMH volume was estimated with fluorochrome-conjugated anti-D in accordance with the manufacturer’s instructions (Fluro-D, CSL, Melbourne, Vic.) and analysed on a Coulter Epics XL flow cytometer (Beckman Coulter, Calif, USA). In brief, the method entailed incubating 2.5 mL of maternal blood with fluorescein isothiocyanate-conjugated anti-D for 15 minutes at room temperature. Controls, consisting of 0, 0.2%, 0.4% and 4% Rh D-positive red cells in Rh D-negative blood, were analysed in parallel with the maternal samples. Red cells were gated by means of forward-scatter and side-scatter, and 100 000 red cell events were analysed. The fluorescing Rh D-positive red cells with bound fluorescein isothiocyanate-conjugated anti-D were determined as a percentage of the total number of red cells analysed. The volume of Rh D-positive red cells in the maternal circulation was then calculated according to the guidelines of the Australian and New Zealand Society of Blood Transfusion, as shown in Box 1.7
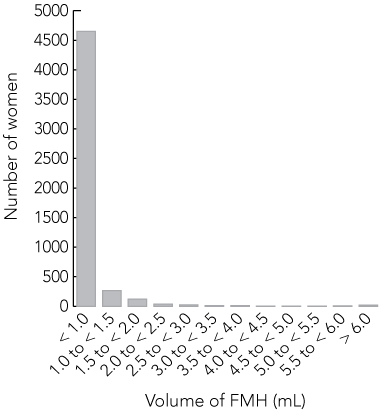
The flow cytometry FMH results for all 5148 samples analysed are shown in Box 2. The vast majority (98.5%; 5072) had an FMH volume of less than 2.5 mL; 90.4% (4651) had a volume of less than 1.0 mL. Only 1.1% (51) had volumes between 2.5 to less than 6.0 mL, and 0.4% (25) had a volume of 6.0 mL or greater (range, 6.0–92.4 mL).
This audit of 5148 FMH volume quantitations provides strong evidence that the amount of prophylactic anti-D currently routinely administered to Rh D-negative women postpartum in most countries, including Australia, is excessive. We thus present evidence on which to base a change in the recommendations for this practice. As highlighted by the Cochrane reviewers, such evidence is currently lacking in the international literature.8 Our article also presents a model system capable of managing the accurate assessment of FMH volume over a sizeable geographical area. Our accumulation of data over such a large geographical area and dispersed population as Western Australia suggests that the implementation of this form of FMH volume quantitation is achievable in other health care systems.
In 1999 in Australia, the ARCBS supplied an average of 15 × 106 IU of anti-D per month for processing by the manufacturer, CSL Bioplasma (Melbourne, Vic). Introduction of antenatal prophylaxis at 28 and 34 weeks’ gestation for Rh D-negative primigravidae in late 2002 required the importation of an anti-D product from Canada for postpartum prophylaxis because there was insufficient domestic product. The implementation of antenatal prophylaxis to all Rh D-negative women has required the ARCBS to increase collection of anti-D to 30 × 106 IU per month. A further 15 × 106 IU anti-D per month would have been required to eliminate the need for importation of postpartum prophylactic anti-D.5 Following increased production of anti-D by the ARCBS, Australia has become self-sufficient in 2006.
A 250 IU anti-D vial for first trimester sensitising events3 was manufactured for use in 2001 to replace the 625 IU dose. This came 6 years after calls for a reduced dose because of critical shortages of anti-D.9 Our retrospective audit shows that this 250 IU (50 mg) dose could also be routinely used for 98.5% of Rh D-negative women postpartum. According to ARCBS data, reducing the postpartum anti-D dose to 250 IU would save an estimated 9 × 106 IU per month.5
Firstly, FMH screening and quantitation could be performed at a centralised laboratory by flow cytometry, as we did in this study. Flow cytometry, in particular with monoclonal anti-D, is well recognised as being the most accurate and reproducible method for FMH volume quantitation.10-12 We also found this approach to be extremely practical over a large geographical area.13
The second approach is the Kleihauer test followed by flow cytometric quantitation. Despite its general lack of reproducibility for FMH volume quantitation, a well-performed Kleihauer test is accepted by many as an appropriate screening technique for detection of FMH.14 Therefore, FMH screening using the Kleihauer test followed by accurate quantitation using flow cytometry if fetal red cells are detected could be considered. Based on our data, we estimate that this approach would necessitate flow cytometric FMH volume quantitation in less than 10% of cases.
Received 6 October 2005, accepted 3 April 2006
- Bradley M Augustson1
- Elizabeth A Fong1
- Dianne E Grey1
- Janine I Davies1
- Wendy N Erber2
- 1 PathWest Laboratory Medicine WA, Perth, WA.
- 2 Addenbrooke's Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, Cambridgeshire, United Kingdom.
Sincere thanks to Dr Gary Hoffman for his critical review of the manuscript and to Professor Ian Jacobs for statistical and epidemiological input.
None identified.
- 1. Mollison PL, Englefriet CP, Contreras M. Haemolytic disease of the foetus and newborn. In: Blood transfusion in clinical medicine. 10th ed. Oxford: Blackwell Science, 1997: 543-591.
- 2. Royal College of Obstetricians and Gynaecologists. Clinical green top guidelines. Use of anti-D immunoglobulin for Rh prophylaxis (22) — revised May 2002. Available at: http://www.rcog.org.uk/index.asp?PageID=512 (accessed May 2006).
- 3. National Blood Authority. Guidelines on the prophylactic use of Rh D immunoglobulin (anti-D) in obstetrics — June 2003. Canberra: NBA, 2003. Available at: http://www.nba.gov.au/pubs.htm (accessed May 2006).
- 4. National Health and Medical Research Council. Guidelines on the prophylactic use of Rh D immunoglobulin (anti-D) in obstetrics [rescinded]. Canberra: NHMRC, 1999. Available at: http://www.nhmrc.gov.au/publications/synopses/wh27syn.htm (accessed May 2006).
- 5. Davison T, Wylie B. Rh(D) immunoglobulin — where does it come from [abstract]? Transfus Med 2005; 15: 75. (Abstract No. 27.)
- 6. Thomson A. Rh D prophylaxis in Australia: 1966 and beyond [abstract]. Transfus Med 2005; 15: 75. (Abstract No. 26.)
- 7. Scientific Subcommittee of the Australian and New Zealand Society of Blood Transfusion. Guidelines for laboratory assessment of fetomaternal haemorrhage. 1st ed. Sydney: ANZSBT, 2002: 3-12.
- 8. Crowther C, Middleton P. Anti-D administration after childbirth for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev 2000; (2): CD000021.
- 9. de Crespigny L, Davison G. Anti-D administration in early pregnancy — time for a new protocol. Aust N Z J Obstet Gynaecol 1995; 35: 385-387.
- 10. Kennedy GA, Shaw R, Just S, et al. Quantification of feto-maternal haemorrhage (FMH) by flow cytometry: anti-fetal haemoglobin labelling potentially underestimates massive FMH in comparison to labelling with anti-D. Transfus Med 2003; 13: 25-33.
- 11. Chen JC, Davis BH, Wood B, Warzynski MJ. Multicenter clinical experience with flow cytometric method for fetomaternal hemorrhage detection. Cytometry 2002; 50: 285-290.
- 12. Johnson PR, Tait RC, Austin EB, et al. Flow cytometry in diagnosis and management of large fetomaternal haemorrhage. J Clin Pathol 1995; 48: 1005-1008.
- 13. Fong EA, Davies JI, Grey DE, et al. Detection of massive transplacental haemorrhage by flow cytometry. Clin Lab Haematol 2000; 22: 325-327.
- 14. Bromilow IM, Duguid JK. Measurement of feto-maternal haemorrhage: a comparative study of three Kleihauer techniques and two flow cytometry methods. Clin Lab Haematol 1997; 19: 137-142.





Abstract
Objective: To assess the potential for dose-reduction of prophylactic anti-D postpartum.
Design: Retrospective audit of fetomaternal haemorrhage (FMH) quantitation by flow cytometry.
Participants and setting: 5148 consecutive Rhesus D-negative women aged 15–45 years who had FMH estimation by flow cytometry at a central laboratory in Western Australia in the 65 months between 1 August 1999 and 31 January 2005.
Main outcome measures: Quantitation of FMH volume for adequate prophylactic anti-D administration in a timely fashion.
Results: 90.4% (4651/5148) of the women had an FMH volume of 1.0 mL or less of Rh D-positive red cells, and 98.5% (5072/5148) had a volume of less than 2.5 mL. Only 0.4% of cases had an FMH volume of 6.0 mL or greater (range, 6.0–92.4 mL).
Conclusions: This large retrospective audit shows that a currently available dose of 250 IU (50 mg) of anti-D would have been sufficient for 98.5% of the 5148 Rh D-negative women. On the basis of this evidence, a reduction in the recommended routine postpartum dose of anti-D from 625 IU to 250 IU when flow cytometric quantitation for FMH is available should be considered. Adopting such a strategy would ensure the ongoing provision of a valuable human blood product currently in limited supply.