Diphtheria and tetanus have been well controlled in Australia since routine childhood vaccination began in the 1950s, but immunity in older Australians may not be optimal.
Diphtheria is caused by toxigenic Corynebacterium diphtheriae, which spreads from person to person via the respiratory route or by direct contact with infected skin lesions. There have been no reported cases of laryngeal diphtheria in Australia since 1991–92, when 23 cases and one death were reported in the Northern Territory, mainly in Aboriginal adults.1 However, a widespread outbreak of diphtheria in Russia and the newly independent states of the former Soviet Union between 1990 and 1997 (140 000 cases and more than 4000 deaths)2 showed the potential for re-emergence of epidemic disease in inadequately immunised populations. During this period, there were small outbreaks associated with imported cases in the United States and Western Europe.3,4 Recent serosurveys in other countries,5,6 as well as pre-vaccination trial data from Sydney,7 indicate that a significant proportion of adults lack adequate protective immunity against diphtheria.
Tetanus usually results from contamination of a wound with soil containing spores of Clostridium tetani, which germinate and release neurotoxin. The organism does not spread from person to person and, contrary to diphtheria, herd immunity plays no role in its control. Tetanus is rare in people who have received a complete vaccination course. In industrialised countries, it usually occurs in those born before routine infant vaccination was introduced.8 In Australia, there have been fewer than 10 notifications of tetanus per annum since 1995, compared with 20–40 recorded hospital admissions for tetanus and occasional deaths.9 This discrepancy probably represents failure of clinicians to notify some cases and incorrect classification or misdiagnosis of hospitalised cases, as the diagnosis is rarely confirmed in the laboratory. Most hospitalised cases and virtually all deaths occur in people over 60 years of age. In one region of New South Wales, a study of tetanus immunity among adults (≥ 49 years) showed that only half had serological evidence of adequate protection.10
We report the results of the first national serosurvey of immunity to tetanus and diphtheria in Australians.
National serosurveys are performed on behalf of the National Centre for Immunisation Research and Surveillance at the Centre for Infectious Diseases and Microbiology using a bank of about 13 000 residual sera collected between July 1996 and May 1999 from 45 of 52 large diagnostic laboratories throughout Australia.
Sera from subjects who were immunocompromised, had received multiple transfusions in the 3 months before the sera sample was taken, or were known to be infected with HIV, were excluded. Sera submitted for measles diagnosis (during evaluation of the Australian Measles Control Campaign 1998) were also excluded.11
Sera were identified at the referring laboratory by sex of the subject, age or date of birth, residential postcode, date of collection and a unique identifier, to ensure that only one sample from any subject was tested. Sera were de-identified before testing and coded by date of collection, state/territory of origin, and referring laboratory.
Serum sample sizes for this study were determined on the basis of expected levels of immunity to tetanus or diphtheria in each of the age groups 5–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, and 70 years and over, to achieve confidence intervals of about ± 5%. Additional sera were tested for tetanus antitoxin in age groups 50 years and over to detect an expected difference between men and women of 10%, if present. Within each age group, states and territories were sampled in proportion to their population size in 1998 (Australian Bureau of Statistics mid-year estimate).12 About equal numbers of sera from men and women were tested except in the 70–95 years age group, in which sample sizes were proportional to the sex distribution in the 1998 population.
Diphtheria and tetanus antitoxin levels were measured using a double antigen enzyme immunoassay (DAEIA), in which one Fab arm of each IgG antibody molecule binds to immobilised toxoid coating the well of a microtitre plate, while the other binds to soluble labelled toxoid to give a signal. Both assays have been shown to correlate well with standard toxin neutralisation assays.13 For both assays, sera were classified as negative (susceptible) when the antitoxin level was < 0.01 International Units (IU)/mL and positive (immune) when it was ≥ 0.1 IU/mL. Antitoxin levels in the range 0.01–< 0.1 IU/mL were classified as low positive (partially immune).
Details of the double antigen enzyme immunoassays are described in Box 1.
Calculated test sample sizes of 1950 sera for diphtheria and 2884 for tetanus were representative of the Australian population by jurisdiction and sex within each age group. About 99% of children aged 5–9 years had at least partial immunity (antitoxin levels ≥ 0.01 IU/mL) to both diphtheria and tetanus (Box 2). Antitoxin levels fell progressively with age and generally more rapidly for diphtheria than tetanus.
By age 20 years, the proportion of subjects with at least partial immunity to diphtheria was below 85% (Box 2). This fell to less than 60% in people over 50 years of age, the age group in which many subjects may not have received routine childhood immunisation. Waning immunity in previously immunised age groups (from 5–9 to 30–49 years) was reflected by increasing proportions of partially immune subjects with age (Box 2).
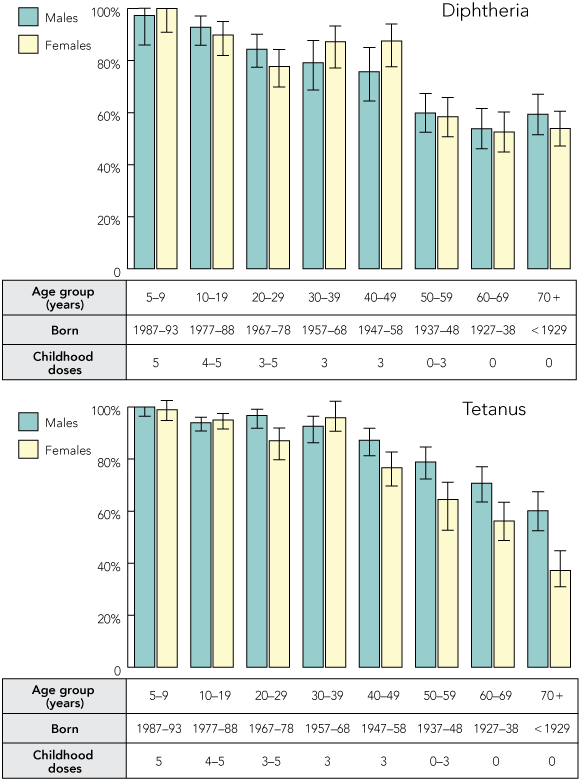
Proportions of males and females with at least partial immunity did not differ significantly in each age group (Box 3) except in 30–49 year olds (87% of females v 77% males, P = 0.02, had levels ≥ 0.01 IU/mL).
As for diphtheria, the proportion of subjects with at least partial immunity (antitoxin levels ≥ 0.01 IU/mL) decreased with age (Box 2). The proportion of subjects with partial immunity was lowest in the age groups 5–9 and 20–39 years and around 20% in other age groups (Box 2). In most age groups, more males than females had at least partial immunity to tetanus (Box 3). The difference was most marked in the age group ≥ 70 years (37% v 60%; P < 0.001), and statistically significant in all age groups over 40 years and in the age group 20–29 years.
This is the first national serosurvey of tetanus and diphtheria immunity in Australia. Over 90% of children had tetanus and diphtheria antitoxin levels ≥ 0.01 IU/mL indicating immunity or partial immunity, but levels decreased significantly with age. Older people, especially women in the case of tetanus, had the lowest antitoxin levels.
These trends reflect past and current vaccination strategies.8,15 The high levels of antitoxin in children indicate good uptake of diphtheria–tetanus–pertussis (DTP) vaccine as part of the childhood vaccination schedule.9 Infant vaccination against diphtheria was introduced in 1940–1945, which may explain the significant decline in immunity between ages 40–49 and 50–59 years. The drop in tetanus immunity to below 90% in 40–49 year olds can probably be explained by the later introduction of infant vaccination against tetanus, as DTP, in 1953. The significantly higher levels of tetanus antitoxin among elderly men, compared with women in the same age group, are probably the result of vaccination during military service in the Second World War, while higher levels in young men may reflect booster doses after tetanus-prone wounds, which are more common in young men than women.
Our results are comparable with those of serosurveys in other countries with similar vaccination histories, such as the United Kingdom, the United States and the Netherlands.5,6,16 They are also consistent with findings of earlier studies in Victoria17,18 and NSW,7,10 which showed that cohorts born before the introduction of mass vaccination had relatively low antitoxin levels. However, direct comparisons between serosurveys can be difficult owing to inter-laboratory and inter-assay variability. To allow comparisons with other countries, our diphtheria antitoxin assay results were standardised against those of a designated reference laboratory in Finland, as part of the European Sero-Epidemiology Network (ESEN2) study. They will be used to assess the impact of changes in vaccination policies and to help determine optimal strategies for the future.
Although low antitoxin levels do not necessarily indicate susceptibility19 and there is no agreed protective tetanus antitoxin level, it is likely that, in elderly Australians, immunity to both tetanus and diphtheria is suboptimal. The fact that most recent cases of tetanus have been in older people supports this conclusion. In addition, many of the recent cases of diphtheria in countries such as the UK20 and the US,3,21 which have similar seroepidemiological profiles to Australia, have been in adults, with most acquiring their infection overseas. Therefore, travellers (especially older adults) to endemic or epidemic regions need to be fully immunised to reduce the risk of reintroducing diphtheria into Australia. If it were re-introduced, an epidemic could occur, as fewer than 75% (the proportion required for herd immunity)22 of adults aged 50 years and over are adequately protected. Nevertheless, childhood immunity is a more important determinant of outbreaks,5 so maintenance of high infant immunisation rates (above 90%)22 is the most effective means of prevention.
To improve immunity in adults, changes to the Australian Standard Vaccination Schedule were recommended in 2000. A booster dose of adult diphtheria/tetanus (dT) vaccine given at age 50 years23 should eventually improve immunity in people now aged 20–49 years. Immunity in young adults should also improve after the introduction in 2003 of a national school-based program providing a booster dose of dTpa (dT with acellular pertussis vaccine) at 15–17 years.15 However, additional efforts will be required to improve immunity in people over 50 years, who are most at risk.
In addition to changes in the Standard Vaccination Schedule for DTP, meningococcus group C and pneumococcal vaccines have been recently introduced in Australia. The protein conjugate in these vaccines is either a mutant diphtheria toxin or tetanus toxoid,15 and their use has been associated with both enhanced and impaired immunity to tetanus and diphtheria.24,25 It is therefore important to monitor tetanus and diphtheria immunity with further serosurveys.
In conclusion, the first national serosurvey for tetanus and diphtheria indicates that immunity in children is good, but adults, especially older people, may not be adequately protected. Maintaining high childhood vaccination rates is necessary to achieve herd immunity to diphtheria and to protect individuals against tetanus. Recent changes to the vaccination schedule should improve immunity in cohorts now aged less than 50 years, but additional efforts will be required to protect those over 50, who are most at risk. To monitor the effects of changes to the vaccination schedule, ongoing serosurveillance is recommended.
1 Measurement of diphtheria and tetanus antitoxin levels using a double antigen enzyme immunoassay (DAEIA)
Diphtheria DAEIA
Microtitre plates (Nunc-Immuno MaxiSorp, Roskilde, Denmark) were coated overnight at 4°C with diphtheria toxoid (1st International Reference Reagent [900 Lf/mL; ie, 900 limit of flocculation units per mL], Diphtheria Toxoid for Flocculation Tests, DIFT, National Institute for Biological Standards and Control [NIBSC], Potters Bar, Herts, UK) diluted to give 0.05 g/well in 50 L carbonate buffer, pH 9.6 (Sigma, St. Louis, Mo, USA). The wells were blocked for 1 hour at 37°C with 100 L StabilGuard Biomolecule Stabilizer (SurModics Inc, Freiburg, Germany) and then aspirated and dried at 37°C in a vacuum oven for 4 hours. Plates were stored until use at 4°C in a plastic bag containing a desiccator strip.
On the day of testing, the plates were allowed to reach room temperature. On each plate, doubling dilutions (1:2 to 1:128) of the reference serum (1st International Standard for Diphtheria Antitoxin, Equine, DI 00/462, NIBSC) 10 IU/mL prediluted to 0.15 IU/mL, and test sera were made in assay buffer (phosphate buffered saline [PBS], 1% v/v Tween 20, 1% bovine serum albumin [BSA]). Plates were incubated overnight at 4°C. After incubation, they were washed four times with wash buffer, (PBS, 1% v/v Tween 20) and 50 L of biotin (Sigma)-labelled diphtheria toxoid, diluted 1:1000 in dilution buffer, was added to each well and incubated at room temperature for 1 hour. The plates were then washed four times and 50 L of streptavidin-horseradish peroxidase (Amersham Pharmacia Biotech Inc, Pitscataway, NJ, USA), diluted 1:1000 in dilution buffer, was added to each well and incubated for 1 hour at room temperature. After washing, 200 L of ortho-phenylenediamine (Sigma) was added to each well, incubated at room temperature for 20 minutes and the reaction stopped by the addition of 50 L of 3 mol/L sulfuric acid. The optical density was read at 492/620 nm using a LP400 (Diagnostics Pasteur) plate reader (Sanofi Diagnostics Pasteur, Chaska, Minn, USA).
Optical density readings for the reference sera were used to produce a standard curve for each plate, and antitoxin concentrations (IU/mL) for the test sera were calculated using a personal computer and Multicalc, version 2.60 (Wallac Oy, Turku, Finland). The assay was also standardised against a panel of sera provided by the European Sero-Epidemiology Network.14
Tetanus DAEIA
The method was identical to that of the diphtheria DAEIA with three exceptions. The microtitre plates were coated with tetanus toxoid (1st International Reference Reagent 1988 [1000 Lf/mL], Tetanus Toxoid for Flocculation Tests, TEFT, NIBSC) diluted to give 0.01 g in 50 L of dilution buffer. The reference serum used was the 1st International Standard for Tetanus Immunoglobulin, Human (TE-3 NIBSC) 120 IU/mL prediluted to 0.1 IU/mL. After the overnight incubation of serum samples, 50 L of biotin-labelled tetanus toxoid was added to each well.
2 Immunity to diphtheria and tetanus by age group
|
Age group (years) |
||||||||||||||
Antitoxin |
5–9 |
10–19 |
20–29 |
30–39 |
40–49 |
50–59 |
60–69 |
≥ 70 |
|||||||
Diphtheria |
|
|
|
|
|
|
|
|
|||||||
Number tested |
76 |
195 |
280 |
155 |
146 |
350 |
348 |
400 |
|||||||
Immune* |
93% |
70% |
51% |
50% |
45% |
33% |
29% |
33% |
|||||||
Partially immune† |
5% |
22% |
30% |
34% |
37% |
26% |
24% |
24% |
|||||||
Immune or partially immune‡ (95%CI) |
99% |
91% |
81% |
83% |
82% |
59% |
53% |
57% |
|||||||
(93%–100%) |
(86%–95%) |
(76%–85%) |
(76%–89%) |
(74%–87%) |
(54%–64%) |
(48%–59%) |
(51%–61%) |
||||||||
Tetanus |
|
|
|
|
|
|
|
|
|||||||
Number tested |
208 |
703 |
245 |
243 |
344 |
371 |
369 |
401 |
|||||||
Immune* |
89% |
72% |
79% |
81% |
65% |
49% |
39% |
25% |
|||||||
Partially immune† |
10% |
22% |
13% |
13% |
17% |
22% |
24% |
22% |
|||||||
Immune or partially immune‡ (95% CI) |
100% |
94% |
92% |
94% |
82% |
72% |
63% |
47% |
|||||||
(97%–100%) |
(92%–96%) |
(88%–95%) |
(91%–97%) |
(78%–86%) |
(67%–76%) |
(58%–68%) |
(42%–52%) |
||||||||
* Immune = ≥ 0.1 IU/mL. †Partially immune = 0.01–< 0.1 IU/mL. ‡Immune or partially immune = ≥ 0.01 IU/mL |
|||||||||||||||
Received 18 May 2005, accepted 18 July 2005
- Heather F Gidding1
- Josephine L Backhouse2
- Gwendolyn L Gilbert3
- Margaret A Burgess4
- 1 Centre for Infectious Diseases and Microbiology (CIDM)-Public Health, Institute of Clinical Pathology & Medical Research (ICPMR), Westmead Hospital, Sydney, NSW.
- 2 National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases (NCIRS), Royal Alexandra Hospital for Children, Sydney, NSW.
We thank the staff of the 45 laboratories who provided the sera (see Australian Measles Control Campaign 1998 Evaluation Report, pages 8–9
None identified.
- 1. Patel M, Morey F, Butcher A, et al. The frequent isolation of toxigenic and non-toxigenic Corynebacterium diphtheriae at Alice Springs Hospital. Commun Dis Intell 1994; 18: 310-311.
- 2. Vitek CR, Wharton M. Diphtheria in the former Soviet Union: reemergence of a pandemic disease. Emerg Infect Dis 1998; 4: 539-550.
- 3. Welty T, La Fromboise C, Dixon J, et al. Toxigenic Corynebacterium diphtheriae — Northern Plains Indian Community, August-October 1996. MMWR Morb Mortal Wkly Rep 1997; 46: 506-510.
- 4. Diphtheria cases notified in the European Union [Editorial Committee]. EuroSurveillance 1997; 2: 63-64.
- 5. Edmunds WJ, Pebody RG, Aggerback H, et al. The sero-epidemiology of diphtheria in Western Europe. ESEN Project. European Sero-Epidemiology Network. Epidemiol Infect 2000; 125: 113-125. (Erratum in: Epidemiol Infect 2001; 126: 331.)
- 6. McQuillan GM, Kruszon-Moran D, Deforest A, et al. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med 2002; 136: 660-666.
- 7. Turnbull FM, Heath TC, Jalaludin BB, et al. A randomized trial of two acellular pertussis vaccines (dTpa and pa) and a licensed diphtheria-tetanus vaccine (Td) in adults. Vaccine 2000; 19: 628-636.
- 8. Gidding HF, Burgess MA, Kempe AE. A short history of vaccination in Australia. Med J Aust 2001; 174: 37-40.
- 9. Brotherton J, McIntyre P, Puech M, et al. Vaccine preventable diseases and vaccination coverage in Australia 2001 to 2002. Commun Dis Intell 2004; 28 Suppl 2: vii-S116.
- 10. Heath TC, Smith W, Capon A, et al. Tetanus immunity in an older Australian population. Med J Aust 1996; 164: 593-596.
- 11. Gilbert GL, Escott RG, Gidding HF, et al. Impact of the Australian Measles Control Campaign on immunity to measles and rubella. Epidemiol Infect 2001; 127: 297-303.
- 12. AusStats. Population by age and sex, Australian states and territories. Canberra: ABS, 1999. (Catalogue No. 3201.0.) Available at: http://www.abs.gov.au/ausstats/abs@.nsf/70e266437a51906dca256820001438b9/f01739c0704ed991ca2569b90082e723!OpenDocument (accessed Jul 2005).
- 13. Kristiansen M, Aggerbeck H, Heron I. Improved ELISA for determination of anti-diphtheria and/or anti-tetanus antitoxin antibodies in sera. APMIS 1997; 105: 843-853.
- 14. von Hunolstein C, Aggerbeck H, Andrews N, et al. European Sero-Epidemiology Network: standardisation of the results of diphtheria antitoxin assays. Vaccine 2000; 18: 3287-3296.
- 15. National Health and Medical Research Council. The Australian immunisation handbook. 8th ed. Canberra: Australian Government Department of Health and Ageing, 2003.
- 16. de Melker HE, van den Hof S, Berbers GA, et al. A population-based study on tetanus antitoxin levels in The Netherlands. Vaccine 1999; 18: 100-108.
- 17. Trinca JC. Immunity to tetanus in Victoria, 1973. Med J Aust 1974; 2: 595-598.
- 18. Forsell P. Diphtheria immunity in Victoria. Med J Aust 1972; 1: 1023-1026.
- 19. Bowie C. Tetanus toxoid for adults — too much of a good thing. Lancet 1996; 348: 1185-1186.
- 20. de Benoist AC, White JM, Efstratiou A, et al. Imported cutaneous diphtheria, United Kingdom. Emerg Infect Dis 2004; 10: 511-513.
- 21. Bisgard KM, Hardy IR, Popovic T, et al. Respiratory diphtheria in the United States, 1980 through 1995. Am J Public Health 1998; 88: 787-791.
- 22. World Health Organization. Diphtheria epidemic in Europe: emergency response. Report on a WHO Meeting; 1993 July; St Petersburg, Russia. WHO Report No. EUR/ICP/EPI 038 Rev 1.
- 23. National Health and Medical Research Council. The Australian Immunisation Handbook. 7th ed. Canberra: AGPS, 2000.
- 24. Olander RM, Wuorimaa T, Kayhty H, et al. Booster response to the tetanus and diphtheria toxoid carriers of 11-valent pneumococcal conjugate vaccine in adults and toddlers. Vaccine 2001; 20: 336-341.
- 25. Hoppenbrouwers K, Roelants M, Ethevenaux C, et al. The effect of reconstitution of an Haemophilus influenzae type b-tetanus toxoid conjugate (PRP-T) vaccine on the immune responses to a diphtheria-tetanus-whole cell pertussis (DTwP) vaccine: a five-year follow-up. Vaccine 1999; 17: 2588-2598.





Abstract
Objective: To determine immunity to tetanus and diphtheria in the Australian population.
Design and setting: Analysis, using double antigen enzyme immunoassays, of a representative sample of sera (1950 samples tested for diphtheria and 2884 for tetanus) collected opportunistically from Australian laboratories between July 1996 and May 1999.
Main outcome measure: Immunity to diphtheria and tetanus, defined as negative (susceptible) when the antitoxin level was < 0.01 IU/mL, positive (immune) when it was ≥ 0.1 IU/mL, and low positive (partially immune) when it was in the range 0.01–< 0.1 IU/mL.
Results: About 99% of children aged 5–9 years had diphtheria and tetanus antitoxin levels ≥ 0.01 IU/mL (immune or partially immune). Antitoxin levels declined with age and generally more markedly for diphtheria than tetanus. For subjects aged 50 years and over, less than 60% were immune or partially immune to diphtheria and less than 75% to tetanus. Men and women had similar diphtheria antitoxin levels, while women had lower levels of tetanus antitoxin compared with men of the same age, with the difference being most marked in the age group ≥ 70 years (37% v 60%; P < 0.001).
Conclusions: Immunity in children appears to be good, but adults, especially older people, may not be adequately protected. Recent changes to the Australian Standard Vaccination Schedule should improve immunity in cohorts now aged < 50 years. However, additional efforts are required to protect those over 50 years (especially travellers), who are most susceptible.