Venous thromboembolism (VTE) affects 1–2 per 1000 people in the general population each year, usually as deep venous thrombosis (DVT) of the leg or pulmonary embolism (PE).1 The incidence increases from 1 in 10 000 for individuals younger than 40 years to 1 in 100 for those older than 60 years.1
The sequelae of venous thrombosis include pulmonary or systemic embolism, post-thrombotic syndrome, and recurrent VTE. Reducing the burden of disease due to VTE requires effective primary prevention, prompt diagnosis, appropriate treatment of acute thrombosis, and effective long-term secondary prevention.
Here we review the risk factors for VTE, the natural history, diagnosis and treatment of DVT, and primary prevention of VTE. The pathophysiology, diagnosis, prognosis, and treatment of PE and unresolved issues in the management of VTE will be addressed in a subsequent article.2
The risk factors for VTE can be classified as either acute provoking or chronic predisposing factors (Box 1).
One-half to two-thirds of all episodes of VTE are provoked by an acute trigger such as recent hospitalisation, trauma, or surgery.3 Among surgical patients, the risk of DVT is highest for hip and knee surgery or major abdominal surgery, and the patient remains at risk for up to 3 months.4
The remaining episodes of VTE are unprovoked, and occur in patients who are presumed to have a chronic inherited or acquired predisposition for thrombosis. In a quarter of all patients, an underlying predisposition cannot be identified by clinical evaluation or laboratory testing.3
Establishing the presence or absence of provoking or predisposing factors for VTE is important for diagnosis and management. Patients with one or more risk factors are more likely to have a positive diagnosis of VTE. Those who are chronically exposed to a predisposing factor or with unprovoked VTE may require extended anticoagulation because of the high risk of recurrence.
The natural history and clinical features of DVT are mainly determined by the site of thrombosis.
Most DVT starts in the calf. In surgical patients, thrombi often begin intra-operatively. In about half of patients, they resolve spontaneously within 72 hours, but in one-sixth of patients they extend to involve the proximal veins.5 Isolated calf DVT is usually asymptomatic and does not commonly cause clinically significant PE. Proximal extension of thrombus is more common in patients with symptomatic (compared with asymptomatic) calf vein thrombus and occurs in a quarter of patients within 1 week of presentation.5 The presence of symptoms, and the extent of proximal progression are harbingers for an increased risk of PE.
Symptoms of DVT (pain, swelling, tenderness, and redness) generally do not develop until there is involvement of the proximal leg veins.5 Massive thrombosis can result in vascular compromise and venous gangrene.
About half of patients with symptomatic proximal DVT have clinically silent PE at the time of diagnosis,6 and 10% have symptomatic PE.7,8 The prevalence of PE increases with age, particularly above 70 years.7,8
Without treatment, a quarter of proximal DVT will propagate during the first 30 days, one-fifth regress, and the rest will remain unchanged.6 Without adequate treatment, half the patients will experience recurrent symptomatic VTE within 3 months.9
With adequate treatment, regression of thrombus occurs during the first week, and recanalisation and resolution of thrombus occurs in about half the patients, generally within the first 3 months.10,11 Resolution of thrombus is less likely in patients with extensive initial thrombosis or cancer. In some cases, thrombus extends into previously unaffected vein segments despite adequate anticoagulation, but this is usually not associated with an increased risk of PE and is of uncertain clinical relevance.11
Post-thrombotic syndrome is characterised by chronic leg pain, swelling, venous stasis, and leg ulcers. It occurs in 20% of patients with symptomatic deep vein thrombosis after 2 years.5
Among patients with a symptomatic proximal DVT who are treated with heparin and 6 months of warfarin, the incidence of recurrent VTE at 2 years is about 10%.12 The risk of recurrence is highest in patients with an unprovoked event, and is twice as high in those with a proximal DVT compared with an isolated distal thrombosis.5
The clinical diagnosis of DVT is unreliable; among those with symptoms and signs of DVT, only 25% have thrombosis confirmed by diagnostic testing.13
Clinical prediction rules have been developed to improve the probability of an accurate diagnosis. The most widely used model classifies patients into a high, moderate, or low probability of DVT (Box 2).14 When coupled with laboratory and radiological imaging tests, this approach is superior to the traditional approach of performing a diagnostic test in all patients with suspected DVT.
D-dimer is a degradation product of cross-linked fibrin, and its plasma levels are high in more than 80% of patients with VTE, depending on the test and the cut-off that is chosen.16 The sensitivities and specificities of different D-dimer assays differ, so the post-test probability for a given patient varies according to the assay used. Clinicians should be aware of these differences so they can interpret the results accordingly.
Elevated D-dimer levels are not specific for VTE. They may occur in patients with malignancy, infection, recent surgery, trauma, or pregnancy. A negative D-dimer test result excludes the diagnosis of DVT in patients with a low pretest probability of disease.16 However, it has limited utility to exclude the diagnosis in hospitalised patients, and individual laboratories must establish their own cut-offs for a D-dimer result that excludes the diagnosis.
Ultrasonography is the most frequently used imaging method for diagnosing DVT because it is accurate for detecting proximal thrombus, non-invasive, and widely available. The features of DVT on ultrasound include lack of compressibility of the vessel lumen, a distended vessel, and lack of flow in the vessel.17 The inability to completely compress the vein lumen is the principal criterion for the diagnosis of DVT.17
The sensitivity and specificity of compression ultrasonography for the diagnosis of symptomatic proximal DVT are more than 95%. For isolated distal DVT, the sensitivity is 70%, and positive predictive value 80%. Therefore, imaging of the calf veins by ultrasonography is generally not recommended; instead the test should be repeated at 1 week to detect any proximal extension of thrombosis that was not evident on the initial study. More recent data suggest that repeat ultrasonography may be avoided if no thrombus is detected by a combination of compression and doppler ultrasonography of the entire leg,18 but this remains to be confirmed.
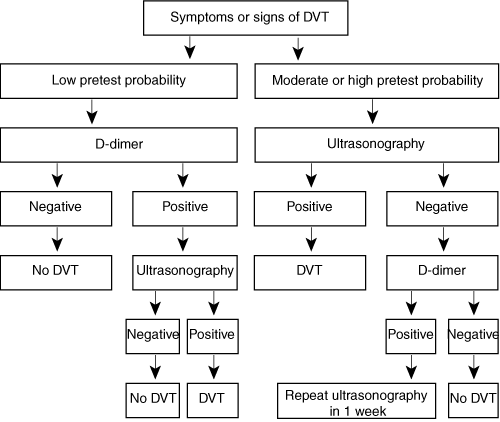
Our recommended approach to the diagnosis of DVT is outlined in Box 3.
Patients with a low clinical pretest probability of a first DVT (probability 5%, based on a score of ≤ 0 from Box 2) should have a D-dimer assay performed initially. A negative D-dimer test result is associated with such a low (0.5%) 3-month cumulative incidence of venous thromboembolism that diagnostic imaging is not required.16 If the D-dimer level is raised, then ultrasonographic scanning is required.17,19 A positive ultrasound result confirms the diagnosis of DVT, and a negative one excludes it (ie, the D-dimer result is likely to have been a false positive).
Patients with a moderate clinical pretest probability (33% prevalence, based on a score of 1–2 from Box 2) or high clinical pretest probability (85% prevalence, based on a score of ≥ 3 from Box 2) should proceed straight to ultrasonography, as a negative D-dimer result cannot reliably exclude the diagnosis. Even with a moderate clinical probability, a negative D-dimer result may be associated with an unacceptably high (3.5%) 3-month incidence of venous thromboembolism.16
A negative compression ultrasound scan in patients with a moderate or high pretest probability does not reliably exclude the diagnosis, as there is still a substantial risk of DVT in the subsequent 3–6 months: 3.6% among patients with moderate probability and 31% among those with high probability.20 A D-dimer assay is indicated. A negative D-dimer result excludes DVT, but a raised level warrants repeat ultrasonography in 1 week.17,19
It may be possible to classify patients into only two groups, according to whether they are “unlikely” or “likely” to have DVT.15 This modified model has been validated in patients with previous thrombosis. When the diagnosis is unlikely and the D-dimer result is negative, ultrasonography can be safely omitted. However, prediction rules must be independently evaluated in different clinical settings because of differences in the prevalence of disease.
Venography is largely limited to patients with negative ultrasonography and unexplained swelling of the entire lower limb, as cases of isolated iliac vein thrombosis may be missed on ultrasonography.19 Venography may also have a role in distinguishing acute recurrent DVT from chronic thrombus, as ultrasonography cannot reliably distinguish between old and new thrombus.17,19
The aims of treatment are to relieve symptoms, reduce the risk of PE or paradoxical embolism to the systemic circulation, prevent post-thrombotic syndrome, and prevent recurrence. The evidence supporting the treatment recommendations has been graded according to the National Health and Medical Research Council’s levels of evidence (Box 4).21
Anticoagulation is the mainstay of treatment for DVT. Contraindications for anticoagulation are summarised in Box 5.22
The initial treatment of acute DVT is with low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) (E1) (Box 6).22,23 LMWH has become the standard of care for the initial treatment of DVT because it is as effective and safe as UFH (E1), but is more convenient to use and has a more favourable side-effects profile. It can be used once or twice daily.
LMWH has a predictable anticoagulant response, so it can be given in a fixed weight-adjusted subcutaneous dose without laboratory monitoring in most patients. This allows out-of-hospital treatment in more than 80% of patients with acute DVT.24 Some patients still require admission to hospital (Box 7). Laboratory monitoring of LMWH is required in patients with renal impairment, obesity, or pregnancy. It has been suggested that, because LMWH is excreted renally, the dose should be halved in patients with a creatinine clearance of less than 30 mL/min (E32). In these patients, UFH may be used instead.
LMWH is less likely than UFH to cause heparin-induced thrombocytopenia (E2).22,23 However, it cannot be used to treat heparin-induced thrombocytopenia because antibodies to UFH cross-react with LMWH. LMWH also causes less osteoporosis than UFH (E2).
UFH requires an initial loading dose followed by continuous intravenous infusion. The anticoagulant response with UFH is unpredictable (because of variable binding of UFH to plasma proteins), and laboratory monitoring of the activated partial thromboplastin time (APTT) is necessary to maintain anticoagulation within the therapeutic range (Box 6) (E2). Because of differences in laboratory reagents and instrumentation, the target therapeutic APTT range must be determined separately in each laboratory, corresponding to an anti-Xa activity of 0.3–0.7 U/mL (E2).25
UFH remains the treatment of choice in patients at high risk of bleeding or undergoing invasive procedures, and in patients with renal failure because of its shorter half-life, reversibility with protamine sulfate, and extra-renal metabolism (E4).
In most cases, warfarin can be started on the first day. LMWH or UFH should be continued for at least 5 days and until the international normalised ratio (INR) has exceeded 2.0 on at least two occasions, 24 hours apart (E2).26 Major bleeding occurs in 1%–5% of patients during initial treatment.27
In patients with extensive ilio-femoral DVT and circulatory compromise, LMWH or UFH should be continued for at least 7 days, and initiation of warfarin should be delayed until anticoagulation has been therapeutic for several days (E4).19
Warfarin (target INR, 2.0–3.0) is the anticoagulant of choice for long-term treatment of most patients with DVT, because it can be given orally and is highly effective, reducing the risk of recurrent VTE by 80%–90% during treatment (E1).28,29
In patients with provoked DVT, the risk of recurrent VTE after discontinuation of warfarin is 1%–4% per year. Generally, warfarin treatment should be continued for 3 months (E2), although there is some evidence that 6 weeks treatment is as effective as 3 months in patients with provoked distal DVT (E2).30
In patients with unprovoked DVT, major chronic predispositions, or active malignancy, the risk of recurrent VTE after discontinuation of warfarin is substantially higher (at least 5%–10% per year) than for provoked events (≤ 4% per year). Decisions regarding the duration of anticoagulant treatment should be individualised by balancing the absolute risk of recurrent VTE (Box 1) with the potential absolute benefits (80%–90% relative risk reduction) and cumulative risks of bleeding (Box 5) associated with anticoagulation.
The optimal intensity and duration of warfarin therapy for VTE are discussed in more detail elsewhere;2 most patients with unprovoked DVT should be treated for at least 6 months (E2), and long-term LMWH therapy is more effective than warfarin to prevent recurrent VTE in patients with cancer (E2).31
Compared with heparin, thrombolysis improves vein patency and reduces the risk of post-thrombotic syndrome, but increases the risk of bleeding, and there is no evidence of a net clinical benefit (E1).32 Thrombolysis and surgical embolectomy have been used as limb-saving therapy in patients with extensive proximal DVT and circulatory compromise or venous gangrene (E4).
Inferior vena cava filters are indicated to prevent pulmonary embolism in patients with DVT who are ineligible for anticoagulant therapy or who experience embolism despite adequate anticoagulation (E4).33 Filters do not obviate the need for anticoagulation because they are associated with an increased risk of recurrent DVT. However, the optimal duration of anticoagulation in patients with vena cava filters in whom anticoagulation is deemed safe is uncertain.
The rationale for routine thromboprophylaxis in high-risk patients is based on the high prevalence of VTE among certain populations, the adverse consequences of VTE, and the proven effectiveness of thromboprophylaxis to reduce VTE and fatal PE.4 Pharmacological thromboprophylaxis increases the risk of bleeding, but clinically important bleeding is uncommon. When appropriately used, pharmacological thromboprophylaxis is cost-effective.
Pharmacological methods must be used with caution in patients undergoing spinal or epidural anaesthesia. Mechanical methods of prophylaxis may be used as an adjunct to pharmacological methods in patients with high or very high risk, and as an alternative to pharmacological methods in patients at high risk of bleeding.4
Recommendations for thromboprophylaxis are summarised in Box 8.
1 Major risk factors for venous thromboembolism
Acute provoking factors
Hospitalisation
Surgery
Trauma or fracture of lower limbs or pelvis
Immobilisation (includes plaster cast)
Long haul travel
Recently commenced oestrogen therapy (eg, within previous 2 weeks)
Intravascular device (eg, venous catheter)
Chronic predisposing factors
Inherited
Inherited or acquired
|
Acquired
|
* Protein C, Protein S, Antithrombin III deficiency. †Chronic cardiorespiratory disease, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders.
2 Assessing the pretest probability of a first episode of deep venous thrombosis14
Clinical feature |
Score |
||||||||||||||
Active cancer (treatment ongoing or within 6 months or palliative) |
1 |
||||||||||||||
Paralysis, paresis or recent plaster immobilisation of the lower extremities |
1 |
||||||||||||||
Recently bedridden for > 3 days, or major surgery within the previous 4 weeks |
1 |
||||||||||||||
Localised tenderness along the distribution of the deep venous system |
1 |
||||||||||||||
Entire leg swollen |
1 |
||||||||||||||
Calf swelling > 3 cm when compared with the asymptomatic leg (at 10 cm below the tibial tuberosity) |
1 |
||||||||||||||
Pitting oedema (greater in the symptomatic leg) |
1 |
||||||||||||||
Collateral superficial veins (non-varicose) |
1 |
||||||||||||||
Alternative diagnosis as likely or greater than that of deep venous thrombosis |
− 2 |
||||||||||||||
In patients with symptoms in both legs, the more symptomatic leg is used. There is a low pretest probability if the score is ≤ 0, moderate if 1–2, and high if ≥ 3. A recent modification has allowed this model to be applied to patients with previous deep venous thrombosis.15 |
|||||||||||||||
4 Level-of-evidence codes21
Evidence for the statements made in this article is graded according to the National Health and Medical Research Council (NHMRC) system for assessing the level of evidence.
E1 Level I: Evidence obtained from a systematic review of all relevant randomised controlled trials.
E2 Level II: Evidence obtained from at least one properly designed randomised controlled trial.
E31 Level III-1: Evidence obtained from well-designed pseudo-randomised controlled trials (alternate allocation or some other method).
E32 Level III-2: Evidence obtained from comparative studies with concurrent controls and allocation not randomised (cohort studies), case–control studies, or interrupted time series without a parallel control group.
E33 Level III-3: Evidence obtained from comparative studies with historical control, two or more single-arm studies, or interrupted time series without a parallel control group.
E4 Level IV: Evidence obtained from case-series, either post-test or pretest and post-test.
5 Contraindications for anticoagulation and risk factors for anticoagulation-associated haemorrhage22
Absolute contraindications
Active bleeding
Relative contraindications
Recent bleeding
Gastrointestinal bleeding within 2 weeks (eg, bleeding peptic ulcer)
Intracranial bleeding within 3 months
Recent major trauma
Bleeding diathesis
Coagulation defect
Severe thrombocytopenia (< 50 × 109/L); inherited or acquired platelet function defect
Uncontrolled hypertension
Endocarditis
Risk factors for anticoagulation-associated haemorrhage
Increasing age
Alcoholism
Cognitive impairment
Chronic corticosteroid use
Liver disease
Peptic ulcer disease
Polymorphisms for the gene encoding the hepatic microsomal enzyme CYP2CP and mutations of Ala-10 in the factor IX propeptide
6 Initial anticoagulation for treatment of deep vein thrombosis
Anticoagulant and dose |
Monitoring* |
Target |
|||||||||||||
Unfractionated heparin |
|||||||||||||||
Loading dose 80 IU/kg; Infusion 18 IU/kg per hour.† |
Measure APTT 6 hours after the bolus dose and adjust infusion accordingly. |
APTT corresponding to anti-Xa 0.3–0.7 U/mL (E2). |
|||||||||||||
Low-molecular-weight heparin‡ |
|||||||||||||||
Dalteparin 100 IU/kg twice daily or 200 IU/kg once daily. |
Renal failure; obesity (eg, > 130 kg); pregnancy (E4). |
Anti-Xa 0.6–1.0 U/mL for twice daily dosing or 1.0–2.0 anti-Xa U/mL for once daily dosing (E4). |
|||||||||||||
Enoxaparin 1 mg/kg twice daily or 1.5 mg/kg once daily. |
|||||||||||||||
APTT = activated partial thromboplastin time. * Platelet counts should be measured twice weekly in all patients to monitor for the development of heparin-induced thrombocytopenia. †Based on the nomogram by Raschke et al. Patients requiring more than 40 000 IU/day of unfractionated heparin to achieve a therapeutic APTT are considered heparin-resistant, and dose should be adjusted according to results of the plasma anti-Xa activity. ‡Dalteparin and enoxaparin are the only low-molecular-weight heparin preparations currently available in Australia. |
|||||||||||||||
7 Indications for admission to hospital for acute deep venous thrombosis (DVT)
High thrombotic load
Massive DVT with circulatory compromise
High risk of bleeding (Box 5)
Role of low-molecular-weight heparin less well defined
Renal failure
Marked obesity (eg, > 130 kg)
Pregnancy
Neonates and children
Safety of home treatment not established
Symptomatic pulmonary embolism
Comorbidities that require hospital admission for treatment (eg, cardiac or respiratory illnesses)
8 Recommendations for thromboprophylaxis
Patient group |
Description* |
Strategy† (level of evidence‡) |
|||||||||||||
Surgical |
Highest risk |
Multiple risk factors; or hip or knee arthroplasty; traumatic hip fracture surgery; or major trauma |
LMWH, warfarin (for hip surgery) plus mechanical methods (E1) |
||||||||||||
|
High risk |
Age > 60 years; age 40–60 years and additional risk factors |
LMWH or low-dose UFH plus mechanical methods (E1) |
||||||||||||
|
Moderate risk |
Minor surgery, additional risk factors; surgery at age 40–60 years and no additional risk factors |
LMWH or low-dose UFH (E1) |
||||||||||||
|
Low risk |
Minor surgery, age < 40 years and no additional risk factors |
No specific prophylaxis required; early mobilisation (E32) |
||||||||||||
Medical |
|
Congestive cardiac failure or severe respiratory disease; confined and at least one additional risk factor |
LMWH or low-dose UFH (E1) |
||||||||||||
Intensive care unit |
Medically ill or postoperative |
LMWH or low-dose UFH (E1) |
|||||||||||||
Long distance travel |
Flights > 6 hours and additional risk factors |
Single dose of LMWH or graduated compression stockings (E31) |
|||||||||||||
* Minor surgery includes operations, other than abdominal surgery, lasting less than 45 minutes; Major surgery includes any intra-abdominal operation and all other operations lasting more than 45 minutes. Additional risk factors include cancer, previous VTE, sepsis, acute neurological disease or inflammatory bowel disease. †Mechanical methods (graduated compression stockings, intermittent pneumatic compression) should be used instead of pharmacological methods in patients with a high risk of bleeding. ‡Levels of evidence according to Box 4. LMWH = low-molecular-weight heparin. UFH = unfractionated heparin. |
|||||||||||||||
- 1. Oger E. Incidence of venous thromboembolism: a community-based study in western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb Haemost 2000; 83: 657-660.
- 2. Lee CH, Hankey GJ, Ho WK, Eikelboom JW. Venous thromboembolism: diagnosis and management of pulmonary embolism. Med J Aust 2005; 182. In press.
- 3. Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002; 162: 1245-1248.
- 4. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126: 338S-400S.
- 5. Kearon C. Natural history of venous thromboembolism. Circulation 2003; 107: I22-I30.
- 6. Nielsen HK, Husted SE, Krusell LR, et al. Anticoagulant therapy in deep venous thrombosis: a randomized controlled study. Thromb Res 1994; 73: 215-226.
- 7. Partsch H, Oburger K, Mostbeck A, et al. Frequency of pulmonary embolism in ambulant patients with pelvic vein thrombosis: a prospective study. J Vasc Surg 1992; 16: 715-722.
- 8. Partsch H, Kechavarz B, Mostbeck A, et al. Frequency of pulmonary embolism in patients who have iliofemoral deep vein thrombosis and are treated with once- or twice-daily low-molecular-weight heparin. J Vasc Surg 1996; 24: 774-782.
- 9. Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301: 855-858.
- 10. Killewich LA, Bedford GR, Beach KW, Strandness DE. Spontaneous lysis of deep venous thrombi: rate and outcome. J Vasc Surg 1989; 9: 89-97.
- 11. Van Ramshorst B, van Bemmelen PS, Hoeneveld H, et al. Thrombus regression in deep venous thrombosis: quantification of spontaneous thrombolysis with duplex scanning. Circulation 1992; 86: 414-419.
- 12. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. N Engl J Med 1995; 332: 1661-1665.
- 13. Wells PS, Hirsh J, Anderson DR, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet 1995; 345: 1326-1330.
- 14. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350: 1795-1798.
- 15. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349: 1227-1235.
- 16. Fancher T, White R, Kravitz R. Combined use of rapid D-dimer testing and estimation of clinical probability in the diagnosis of deep vein thrombosis: systematic review. BMJ 2004; 329: 821-828.
- 17. Fraser JD, Anderson DR. Deep venous thrombosis: recent advances and optimal investigation with US. Radiology 1999; 211: 9-24.
- 18. Stevens SM, Elliott CG, Chan KJ, et al. Withholding anticoagulation after a negative result on duplex ultrasonography for suspected symptomatic deep venous thrombosis. Ann Intern Med 2004; 140: 985-991.
- 19. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002; 93: 3102-3110.
- 20. Michiels JJ, Kasbergen H, Oudega R, et al. Exclusion and diagnosis of deep vein thrombosis in outpatients by sequential noninvasive tools. Int Angiol 2002; 21: 9-19.
- 21. National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, 1999.
- 22. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep vein thrombosis. N Engl J Med 2004; 351: 268-277.
- 23. Hirsh J, Raschke R. Heparin and low-molecular-weight heparin. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126: 188S-203S.
- 24. Eikelboom J, Baker R. Routine home treatment of deep vein thrombosis is now a reality. BMJ 2001; 322: 1191-1193.
- 25. Levine MN, Hirsh J, Gent M, et al. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med 1994; 154: 49-56.
- 26. Hull RD, Raskob GE, Rosenbloom D, et al. Heparin for 5 days as compared with 10 days in the initial treatment of proximal venous thrombosis. N Engl J Med 1990; 322: 1260-1264.
- 27. Levine MN, Raskob G, Beyth RJ, et al. Hemorrhagic complications of anticoagulant treatment. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126: 287S-310S.
- 28. Kearon C. Long term management of patients after venous thromboembolism. Circulation 2004; 110 (Suppl 1): I10-I18.
- 29. Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126: 204S-233S.
- 30. Pinede L, Ninet J, Duhaut P, et al. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001; 103: 2453-2460.
- 31. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349: 146-153.
- 32. Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2004; 4: CD002783.
- 33. Streiff MB. Vena caval filters: a comprehensive review. Blood 2000; 95: 3669-3677.
- 34. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997; 349: 759-762.





Abstract
Venous thromboembolism (VTE) affects 1–2 per 1000 people in the general population each year.
Clinical diagnosis of deep venous thrombosis (DVT) is unreliable, and must be confirmed by compression ultrasonography or venography.
A low clinical pretest probability of DVT and negative D-dimer result reliably exclude the diagnosis, with no need for diagnostic imaging.
Initial treatment of DVT is with low-molecular-weight heparin or unfractionated heparin for at least 5 days, followed by warfarin (target INR, 2.0–3.0) for at least 3 months.
A vena cava filter is indicated in patients who are ineligible for anticoagulant therapy or who experience embolism despite therapeutic anticoagulation.
Thrombolysis or surgical embolectomy may be used as a limb-saving measure in patients with extensive proximal DVT and circulatory compromise that threatens the viability of the leg.
Decisions regarding the optimal duration of anticoagulation to prevent recurrent VTE should be individualised and balance the risk of recurrence if warfarin is stopped against the risk of major bleeding and inconvenience of continuing treatment.
The risk of recurrence is highest in people with recurrent unprovoked DVT or chronic predisposing factors (eg, cancer) who require indefinite anticoagulant treatment.