The World Health Organization (WHO) has defined an adverse drug reaction (ADR) as a “response to a medicine that is noxious and unintended, and that occurs at doses normally used in humans”.1 Iatrogenic factors that lead to ADRs can include inappropriate dosages, inappropriate combinations of drugs, and use of drugs not recommended for a particular age group.2 However, most ADRs occur in patients who are prescribed treatment within the limits of accepted clinical practice.
A 1994 study found that the rate of ADR-related hospital stays in people aged 70 years and over in Western Australia doubled between 1980 and 1991, with the main classes of drugs responsible being cardiovascular agents, antirheumatics and cytotoxics.3 Since then, several Australian Government education and service initiatives have endeavoured to reduce ADRs in older Australians. These include the Quality Use of Medicines Program and the National Prescribing Service.4,5 While the hospital morbidity rate from ADRs is not the only basis on which to evaluate such programs, it offers one criterion by which to judge their success.
This study examines recent trends in ADR-related hospital stays in people aged 60 years and over in WA to determine if these trends and the profile of drugs responsible have changed.
The study population comprised people aged 60 years or over residing in WA between 1981 and 2002. Census and intercensal population estimates by sex and age group were obtained from the Australian Bureau of Statistics.
Hospital stays related to ADRs were identified using the WA Hospital Morbidity Data System, a population-based statutory register which covers all separations (transfers, discharges and deaths) from public and private hospitals in WA.
ADR-related hospital stays were defined as any separation with an “external cause” coded as an adverse event caused by drugs, medicaments or biological substances in therapeutic use. The external cause was usually the cause of the principal condition managed during the hospital stay. In most cases, this condition was present at the time of admission, but it could also arise after admission if it substantially extended the length of stay. Less serious side effects observed on admission or arising in hospital were not included as ADRs in this study.
Separations were coded according to the Manual of the international statistical classification of diseases, injuries and causes of death, ninth revision (ICD-9)6 (1981 to 1987), ICD-9-CM7 (1988 to June 1999) or lCD-10-AM8 (July 1999 to 2002). ADRs were defined as codes E930–E949 (ICD-9 and ICD-9-CM) and Y40–Y59 (ICD-10-AM). They excluded accidents in the technique of administration of drugs. Thus, the definition of an ADR used in the study was consistent with that of the WHO.1
ADR-related stays were identified for the period 1981 to 2002, and were categorised by sex, 10-year age group and responsible drug category.
From 1981 to 2002, 74 380 inpatient stays (0.8% of all stays) were associated with ADRs in the total WA population, of which 43 380 (58.3%) were in people aged 60 years and over.
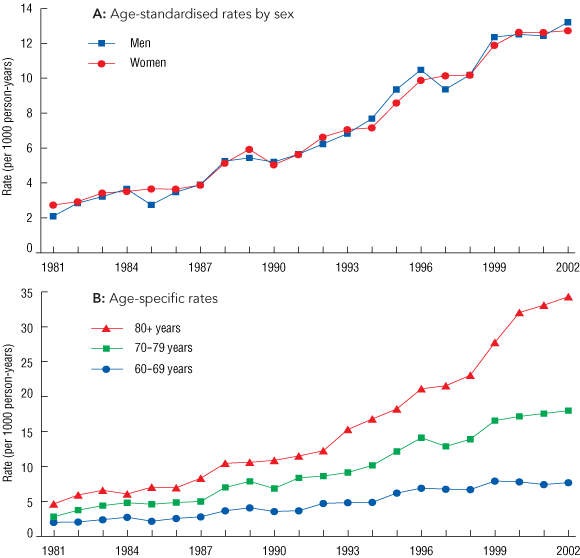
Events in people aged under 60 years were excluded from further analysis. Among older people, 24 553 ADR-related stays were in women (56.6%) and 18 827 in men (43.4%). The rate of ADR-related stays increased sharply with age, rising from 7.7 per 1000 person-years (py) at ages 60–69 years to 34.3 per 1000 py at ages 80 years and over in 2002.
Box 1A shows that the age-standardised rates increased more than fivefold during the study, from 2.5 per 1000 py in 1981 (men, 2.1; women, 2.7 per 1000 py) to 12.9 per 1000 py in 2002 (men, 13.2; women, 12.7 per 1000 py). These rates had a standard error no higher than 0.001 per 1000 py, and a relative standard error no higher than 0.03%. The largest proportional and absolute increases occurred in people aged 80 + years (Box 1B), with an almost tenfold increase in men and a sevenfold increase in women. We estimated that the 25-year risk of an ADR causing admission to or extended stay in hospital from ages 60 to 85 years was a probability of 36% in men and 34% in women.
The drug categories most frequently associated with admission to or extended stay in hospital (Box 2) were cardiovascular agents (17.5%); analgesics, antipyretics and antirheumatics, including non-steroidal anti-inflammatory drugs (NSAIDs) (16.5%); agents affecting blood constituents, including anticoagulants (9.0%); and antibiotics (9.0%). ADRs involving cardiovascular agents were strongly associated with age, with this drug group responsible for 21.7% of ADR-related hospital stays at age 80 + years, compared with 17.9% at age 70–79 and 12.5% at age 60–69 years.
Analysis of more specific drug classes, at the level of 4-digit E-codes (ICD-9 and ICD-9-CM) and 3-digit Y-codes (ICD-10-AM), revealed variation in the drugs involved across age groups. Most common were cytotoxics (13.3%) and corticosteroids (8.0%) at ages 60–69 years; anticoagulants (8.6%) and cytotoxics (7.2%) at 70–79 years; and cardiovascular agents (8.2%) and antirheumatics (8.1%) at 80 + years.
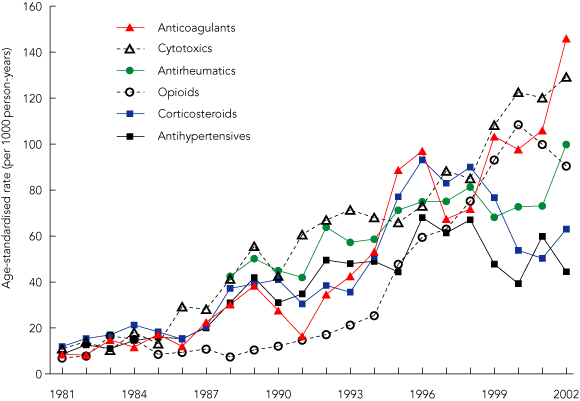
For the entire study population, anticoagulants (7.5%), cytotoxics (7.4%), antirheumatics including NSAIDs (6.8%), corticosteroids (5.8%), opioids (5.6%) and antihypertensives (5.5%) accounted for over a third of all cases. Box 3 shows the trends in age-standardised hospital stay rates from each of these six leading drug categories. The rates of ADRs from corticosteroids and antihypertensives peaked in 1996 and later declined. Adverse events from opioids peaked in the year 2000, whereas those from anticoagulants, cytotoxics and antirheumatics were still rising at the end of the study.
Rates of ADR-related hospital stays continued to increase between 1991 and 2002, with rates doubling since the earlier study in 1991.3 The increase was especially marked in those aged 80 years and over, where cardiovascular agents continue to be the largest problem. The profile of responsible drugs has changed, with an increase in ADRs from anticoagulants and a continued rise in those from NSAIDs and other antirheumatics.
The study was limited to ADRs of sufficient severity to warrant or extend hospitalisation. Thus, the results represent only the extreme end of a continuum of ADRs, most of which occur in the community and are managed on an ambulatory basis. Unfortunately, although the burden of ADRs managed in the community is likely to be significant,9,10 reliable estimates of rates of these ADRs were unavailable. The study was also unable to distinguish ADRs leading to hospitalisation from those arising during inpatient stay and serious enough to become the principal condition managed.
Another limitation was imposed by the necessary reliance on hospital morbidity classification systems and coding practices, which may have changed over time. In the only available validation study, Dawes found that her review of 377 hospital charts was consistent with a real increase in hospital morbidity caused by ADRs, although the administrative hospital data tended to underascertain cases.3
A previous review of 14 Australian studies found that ADRs occurred in 2.4%–3.6% of hospital admissions.9 ADRs were associated with 1.8% of 14 000 admissions to 28 hospitals in New South Wales and South Australia.11 We found that 0.8% of hospital stays in the total WA population were ADR related. Apart from real differences in incidence rates across time and place, results have varied because of bias from hospital selection and differences in ascertainment criteria. Some studies counted any ADR observed at admission or during hospital stay, whereas, along with others, we focused on hospital admissions where the ADR was sufficiently severe to cause the admission or extend hospital stay.
The Pharmaceutical Benefits Scheme (which subsidises about three-quarters of prescriptions dispensed in Australia) paid benefits for 155 million prescriptions in 2001–2002, an increase of 4.7% over 2000–2001.12 This increase exceeded population growth, suggesting either a larger population at risk or a higher average level of drug exposure. Our results suggest that the factors responsible for the increasing incidence rate of ADRs have combined with the ageing of the population, as it is well established that risks of side effects are much higher in older age and worsened by polypharmacy.13
Anticoagulants were the most common drugs implicated in ADRs causing hospitalisation in 2002 and had undergone the greatest increase over time. In 1996, draft guidelines were prepared by the National Health and Medical Research Council (NHMRC) on the role of anticoagulants in stroke prevention.14 Many substances interact adversely with anticoagulants. Older adults are more susceptible to these interactions, have a higher risk of haemorrhage, and therefore require lower anticoagulant doses and careful monitoring. In addition, the increasing use of alternative or complementary therapies raises the potential for adverse interactions with anticoagulants.15
Chemotherapy has become better tolerated because of advances in the management of side effects. Opposing this are the increasing number of cytotoxic agents in use and the increase in active prevalence of cancer in the WA population — from 0.51% in 1990 to 0.74% in 1998 — because of longer survival of patients with active disease.16
NSAID use continues to be a major reason for hospital admission, despite evidence from previous studies indicating the large size of the problem.3,9,17,18 Celecoxib (Pharmacia), an NSAID introduced to the market in 1999, was seventh among the top 10 most prescribed drugs in Australia in 2000 (Box 4).19 Elderly patients taking NSAIDS are at increased risk of gastrointestinal ulceration and renal impairment.17
Cardiovascular agents have been consistently identified as the drug group that causes the most ADRs.10,11,17,18 The antihypertensives enalapril and amlodipine held ninth and tenth positions among Australia’s top 10 most prescribed drugs in 2002 (Box 4). In fact, there was a fall from 1996 in ADR admissions from antihypertensives, suggesting that they no longer warrant urgent attention. Although the lipid-lowering agents atorvastatin and simvastatin were the first and third most frequently used drugs (Box 4), this group is seldom implicated in ADRs causing hospital admissions.
Our findings have policy implications. It has been estimated that at least 80 000 hospital admissions each year in Australia are medication related, at an annual cost of $350 million.9 Despite significant investment of resources by the Australian Government in targeted interventions to empower prescribers, pharmacists and consumers, efforts to reduce the burden of ADRs appear to be faltering against the increasing use of a wider range of medications, especially in older Australians. Arguably, the situation would be worse with no interventions, but our results bring into question their effectiveness and the adequacy of funding.
Particular drug classes, especially anticoagulants, warrant updated clinical guidelines, with increased attention to careful patient monitoring. The NHMRC and the National Medication Safety Breakthrough Collaborative of the Australian Council for Quality and Safety in Health Care provide opportunities to support new initiatives and related needs, such as research into the role of alternative therapies in adverse drug interactions.
Concerns over the rising costs of ADRs in the United States have led to updating of the criteria for potentially inappropriate medication use in older adults.20 The new criteria identify 48 classes of medication to avoid in older adults and a further 20 assessed as potentially inappropriate in the presence of certain comorbid conditions.20 The adoption of such explicit criteria presents a challenge for continuing professional education, behaviour change and medical informatics.
The medical data linkage system in WA is being enhanced by the addition of links between the Pharmaceutical Benefits Scheme and Medicare benefits, paid to or on behalf of WA residents, and the hospital morbidity, cancer, congenital anomaly and death data collections. The system will provide infrastructure for postmarketing surveillance for serious and longer-term ADRs, more detailed characterisation of patients at high risk of ADRs, and an accurate baseline for the evaluation of further intervention.
Ultimately, the risk of side effects must be balanced against the benefits to patients of the increased use of powerful pharmaceutical agents in treating diseases. Even the most well-resourced and carefully designed intervention program imaginable will not prevent all ADRs.
1 Rates of hospital stays related to adverse drug reactions in people aged 60 + years in Western Australia
2 Drugs responsible for hospital stays from adverse drug reactions in Western Australia, 1981–2002 (percentage of reactions in this age group)
Age group (years) |
|||||||||||||||
Drug category |
60–69 (n = 12 505) |
70–79 (n = 17 281) |
80 + (n = 13 594) |
Total (n = 43 380) |
|||||||||||
Agents affecting cardiovascular system |
12.5% |
17.9% |
21.7% |
17.5% |
|||||||||||
Analgesics, antipyretics and antirheumatics* |
15.4% |
16.0% |
18.1% |
16.5% |
|||||||||||
Antibiotics |
9.8% |
8.4% |
9.1% |
9.0% |
|||||||||||
Agents primarily affecting blood constituents |
9.0% |
10.2% |
7.5% |
9.0% |
|||||||||||
Hormones and synthetic substitutes |
11.3% |
9.1% |
6.1% |
8.8% |
|||||||||||
Primarily systemic agents |
13.8% |
7.7% |
2.6% |
7.9% |
|||||||||||
Water, mineral and uric acid metabolism drugs |
5.2% |
7.0% |
10.9% |
7.7% |
|||||||||||
Antidepressants and other psychotropic agents |
5.4% |
5.8% |
6.8% |
6.0% |
|||||||||||
Anticonvulsants and antiparkinsonism drugs |
4.0% |
4.2% |
3.4% |
3.9% |
|||||||||||
Drugs affecting autonomic nervous system |
2.1% |
2.9% |
4.1% |
3.0% |
|||||||||||
Agents affecting skin, eyes and ears |
1.7% |
1.8% |
1.6% |
1.7% |
|||||||||||
Other anti-infectives |
1.7% |
1.4% |
1.3% |
1.5% |
|||||||||||
Anaesthetics and other CNS depressants |
1.6% |
1.2% |
1.0% |
1.3% |
|||||||||||
Agents acting on muscles and respiratory system |
1.3% |
0.9% |
0.9% |
1.0% |
|||||||||||
Agents affecting gastrointestinal system |
0.9% |
0.7% |
1.0% |
0.9% |
|||||||||||
Sedatives and hypnotics |
0.6% |
0.8% |
1.1% |
0.8% |
|||||||||||
CNS stimulants |
< 0.1% |
< 0.1% |
0.1% |
0.1% |
|||||||||||
Bacterial vaccines |
< 0.1% |
0.1% |
< 0.1% |
0.1% |
|||||||||||
Other drugs and medicaments |
3.4% |
3.6% |
2.6% |
3.2% |
|||||||||||
Other vaccines and biological substances |
0.2% |
0.2% |
0.1% |
0.2% |
|||||||||||
CNS = central nervous system. * Including non-steroidal anti-inflammatory agents. |
|||||||||||||||
3 Drug-specific rates of hospital stays from adverse drug reactions in people aged 60 + years in Western Australia
4 The 10 most prescribed therapeutic drugs attracting pharmaceutical benefits in Australia in 2000*
Rank |
Drug |
Defined daily dose (per 1000 person-days) |
|||||||||||||
1 |
Atorvastatin |
Lipid-lowering agent |
39.2 |
||||||||||||
2 |
Salbutamol |
Bronchodilator for asthma |
30.1 |
||||||||||||
3 |
Simvastatin |
Lipid-lowering agent |
29.7 |
||||||||||||
4 |
Frusemide |
Diuretic |
22.1 |
||||||||||||
5 |
Ranitidine |
H2-receptor antagonist for dyspepsia |
19.0 |
||||||||||||
6 |
Budesonide |
Inhaled corticosteroid |
17.7 |
||||||||||||
7 |
Celecoxib |
Non-steroidal anti-inflammatory drug |
16.4 |
||||||||||||
8 |
Ipratropium bromide |
Anticholinergic for asthma |
16.4 |
||||||||||||
9 |
Enalapril |
ACE inhibitor antihypertensive |
15.6 |
||||||||||||
10 |
Amlodipine |
Calcium-channel blocker antihypertensive |
15.7 |
||||||||||||
ACE = angiotensin-converting enzyme. * Source: Commonwealth Department of Health and Ageing.19 |
|||||||||||||||
Received 30 June 2004, accepted 11 November 2004
- Christel L Burgess1
- C D’Arcy J Holman2
- Anthony G Satti3
- 1 School of Population Health, University of Western Australia, Perth, WA.
- 2 Health Information Centre, Department of Health, Perth, WA.
None identified.
- 1. WHO Collaborating Centre for International Drug Monitoring. The global intelligence network for benefits and risks in medical products. Uppsala: WHO Uppsala Monitoring Centre, 2003. Available at: www.who-umc.org/defs.html (accessed Aug 2003).
- 2. Gallagher LP. The potential for adverse drug reactions in elderly patients. Appl Nurs Res 2001; 14: 220-224.
- 3. Dawes VP. Poisoning in Western Australia: overview, investigation of therapeutic poisoning in the elderly [MPH dissertation]. Perth: University of Western Australia, 1994. Abstract available at: www.populationhealth.uwa.edu.au/dawes1994 (accessed Jun 2004).
- 4. Commonwealth Department of Health and Ageing. National Medicines Policy — Quality Use of Medicines. Canberra: The Department, 2002.
- 5. Commonwealth Department of Health and Ageing. National Medicines Policy – National Prescribing Service. Health Access and Financing Division. Canberra: the Department, 2003.
- 6. World Health Organization. Manual of the international statistical classification of diseases, injuries and causes of death, 9th revision. Geneva: WHO, 1977.
- 7. National Coding Centre. Australian version of the international classification of diseases, 9th revision, clinical modification (ICD-9-CM). Sydney: National Coding Centre, 1995.
- 8. National Centre for Classification in Health. The international statistical classification of diseases and related health problems, 10th revision, Australian modification (ICD-10-AM). Sydney: National Centre for Classification in Health, 1999.
- 9. Roughead EE. The nature and extent of drug-related hospitalisations in Australia. J Qual Clin Pract 1999; 19: 19-22.
- 10. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003; 289: 1107-1016.
- 11. Wilson RM, Runciman WB, Gibberd RW, et al. The quality in Australian health care study. Med J Aust 1995; 163: 458-471. <eMJA pdf>
- 12. Commonwealth Department of Health and Ageing. Cost to government of pharmaceutical benefits. Health Access and Financing Division. Canberra: The Department, 2003.
- 13. Gibian T. Rational drug therapy in the elderly or how not to poison your elderly patients. Aust Fam Physician 1992; 21: 1755-1760.
- 14. Donnan G. National Health and Medical Research Council releases draft clinical practice guidelines for stroke prevention – the role of anticoagulants, antiplatelets and carotid endarterectomy. Canberra: NHMRC, 1996.
- 15. Siahpush M. Why do people favour alternative medicine? Aust N Z J Public Health 1999; 23: 266-271.
- 16. Brameld KJ, Holman CDJ, Threllfall TJ, et al. Increasing ‘active prevalence’ of cancer in Western Australia and its implications for health services. Aust N Z J Public Health 2002; 26: 164-169.
- 17. Dartnell JG, Anderson RP, Chohan V, et al. Hospitalisation for adverse events related to drug therapy: incidence, avoidability and costs. Med J Aust 1996; 164: 659-662.
- 18. Chan M, Nicklason F, Vial JH. Adverse drug events as a cause of hospital admission in the elderly. Intern Med J 2001; 31: 199-205.
- 19. Commonwealth Department of Health and Ageing. Australian statistics on medicines 1999-2000. Canberra: The Department, 2003.
- 20. Fick DM, Cooper JW, Wade WE, et al. Updating the Beers Criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003; 163: 2716-2724.





Abstract
Objective: To examine trends in adverse drug reactions (ADRs) in people aged 60 years or over causing admission to or an extended stay in Western Australian hospitals between 1981 and 2002.
Design and setting: Secondary data analysis of case series.
Patients: 43 380 patients admitted to WA public and private hospitals with an (International Classification of Diseases) ICD external cause code for an ADR, identified by the population-based WA Hospital Morbidity Data System.
Main outcome measures: Age-specific, age-standardised and drug-specific rates of ADR-related hospital stays.
Results: The age-standardised rate of ADR-related hospital stays increased from 2.5 per 1000 person-years (py) in 1981 to 12.9 per 1000 py in 2002. The largest increases occurred in those aged 80 + years (tenfold in men and sevenfold in women). The most common drug group involved was cardiovascular agents (17.5%), while anticoagulants (7.5%), cytotoxics (7.4%) and antirheumatics (6.8%) were the more specific drug classes most often implicated. ADRs from the last three classes of drugs were still rising at the end of the study, whereas ADRs from corticosteroids and antihypertensives peaked in 1996 and from opioids in 2000.
Conclusions: Increases in hospital admissions or extended lengths of stay due to ADRs in WA have continued despite programs to promote rational and safer use of medicines. The sharp increase in ADRs from anticoagulants warrants attention to revised clinical guidelines.