A limited number of drugs are available for malaria protection, particularly for long-term prophylaxis. In 2001, doxycycline, mefloquine and Malarone (a combined preparation of atovaquone and proguanil) were recommended by the World Health Organization and the US Centers for Disease Control and Prevention for non-immune travellers in malarious areas where chloroquine-resistant Plasmodium falciparum malaria is prevalent.1,2 Like travellers, military personnel must also take chemoprophylaxis in malarious areas, to minimise non-battle casualties.
In 1999, the Australian Defence Force (ADF) participated in an international peacekeeping operation in East Timor. During the first 5 months of the operation, 64 soldiers presented with malaria in-country.3 Most soldiers had been prescribed daily doxycycline (100 mg) for prophylaxis, and these cases are believed to have resulted from poor compliance. These findings provided the stimulus to look at other chemoprophylactic options for soldiers in East Timor. During 2000–2001, a double-blind trial comparing weekly tafenoquine and mefloquine was conducted in Australian soldiers in East Timor.4 Mefloquine was found to be well tolerated and accepted by the soldiers, and, as a result, there were requests for wider use of mefloquine from subsequent military units and soldiers being deployed to East Timor.
There are limited data on the tolerability of mefloquine for long-term prophylaxis in military personnel. Short-term studies (ranging from 2 to 5 months) in British, Dutch, Indonesian, Italian and US soldiers have shown weekly mefloquine to be safe and well tolerated.5-9 To expand on our previous study in East Timor,4 we monitored the tolerability of mefloquine in a larger number of Australian soldiers under peacekeeping conditions during two 6-month periods. We report here our preliminary findings.
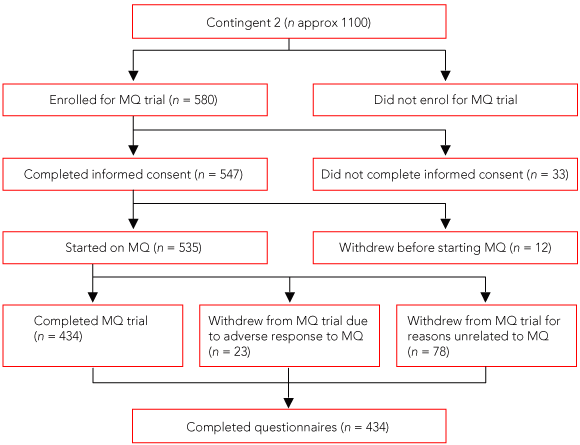
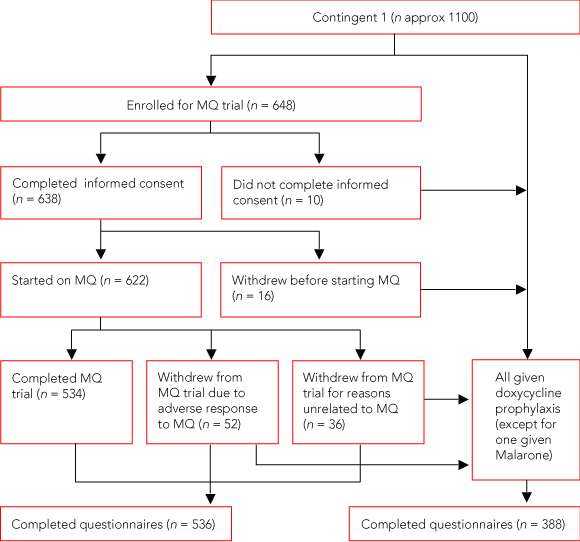
Of the 1228 soldiers who enrolled in the trial (648 in the first contingent and 580 in the second contingent), 1185 provided informed consent, of whom 1157 (1155 men, 2 women) started to take mefloquine (Box 1 and Box 2). Those who enrolled but did not start mefloquine prophylaxis either were not deployed for other reasons or chose not to continue after enrolment. Of the 1157 soldiers who started on mefloquine, 16.3% (189/1157) did not complete their deployment on mefloquine, and 6.5% (75/1157) had adverse responses to the drug. The withdrawal rate from mefloquine prophylaxis due to adverse effects of the drug was higher in the first contingent than in the second (8.4% v 4.3%). The body systems affected, as reported by soldiers who withdrew from mefloquine prophylaxis, are listed in Box 3. All soldiers who stopped taking mefloquine were given doxycycline instead (or Malarone, if they were doxycycline intolerant). Out of the first contingent, 388 soldiers were questioned about the tolerability of doxycycline prophylaxis during their deployment.
At the conclusion of the first contingent’s deployment, 924 soldiers received health questionnaires, including 536 who had taken mefloquine chemoprophylaxis and 388 who had taken doxycycline. Of this group, 57% of soldiers reported one or more adverse events during their use of mefloquine compared with 56% of soldiers using doxycycline. Sleep disturbance, headache, tiredness and nausea were the most commonly reported adverse events (Box 4). A detailed report of adverse events, including data on the second contingent, will be published elsewhere.
Of the 968 soldiers still taking mefloquine at the end of their 6-month deployment, 96% and 92% from the first and second contingents, respectively, indicated that they would take mefloquine on their next deployment to a malarious area. Of the 388 soldiers in the first contingent who were questioned after using doxycycline, 89% indicated they would use it again on deployment.
In our study, the most common adverse events relating to malaria prophylaxis with either drug were sleep disturbance, headache, tiredness and nausea. Apart from mild sleep disturbance, which was more common in soldiers taking mefloquine, and mild tiredness, which was more commonly associated with doxycycline, the incidence of these adverse events was similar for both drugs.
Among the 1157 Australian soldiers taking mefloquine in our study, the 6.5% withdrawal rate from the drug due to adverse events was higher than among Italian soldiers on peacekeeping duties in Africa (0.9% of 1386),8 British soldiers exercising in Kenya (3.4% of 183)5 and US Marines stationed in Hawaii, USA (4.9% of 202).9 The emotional and environmental pressures of peacekeeping operations in East Timor may have contributed to the higher withdrawal rate in the Australian soldiers compared with other military experiences with mefloquine.
In previous studies of mefloquine in military volunteers, no serious neuropsychiatric reactions were observed.5-9 We observed three serious adverse events of this nature that were possibly associated with mefloquine use. This is a higher incidence than the 1 in 6000 to 1 in 10 600 reported in travellers.1 However, two of the three soldiers involved had undisclosed pre-existing conditions that are contraindications for mefloquine use.
When monitoring the tolerability of a drug under military operational conditions, there is a need to account for the physiological and psychological stress associated with such activities that may confound the relationship between drug intake and adverse events. Thus, the tolerability of antimalarial drugs as assessed in these Australian soldiers may not be directly comparable to circumstances of recreational travel. Nevertheless, the withdrawal rate from mefloquine in our study was comparable to that reported in civilian travellers (range, 5%–6.5%).10,11
Furthermore, the cohorts in our study were not randomly allocated and therefore comparisons made may be biased. Our results are also subject to the limitations of self-report and memory.
Despite the possibility of side effects associated with mefloquine (and other antimalarial drugs), this drug protects against potentially life-threatening infections that may also jeopardise the success of military operations. Malaria is endemic in East Timor, and, at the time of the first contingent’s deployment, the prevalence of malaria among East Timorese in the area of deployment was as high as 35%.12 The fact that a soldier not complying with doxycycline use developed malaria in East Timor, as did a small proportion of soldiers after returning to Australia, suggests that the participants were exposed to malaria infections in East Timor and that mefloquine was effective as a suppressive agent against blood stages of both falciparum and vivax malaria.
In conclusion, our study has been one of the largest tolerability trials of mefloquine and doxycycline conducted in military personnel. While the comparison must be interpreted cautiously in light of possible selection bias, it may be concluded that these drugs were generally well tolerated. As Australian soldiers will continue to exercise in and be deployed to malaria-endemic areas, there is a continuing need to seek out effective and well tolerated antimalarial drugs and to maintain alternative chemoprophylactic options. Enhanced surveillance of alternative antimalarial drugs under operational conditions will ensure that the most appropriate chemoprophylaxis is available for the ADF.
3 Adverse events, by body system, reported among Australian soldiers who withdrew from the mefloquine trial due to adverse effects of the drug*
Body system |
First contingent withdrawals (n = 52) |
Second contingent withdrawals (n = 23) |
|||||||||||||
Gastrointestinal |
6 |
4 |
|||||||||||||
Constitutional |
9 |
9 |
|||||||||||||
Neuropsychiatric |
42 |
20 |
|||||||||||||
Dermatological |
3 |
3 |
|||||||||||||
Musculoskeletal |
2 |
0 |
|||||||||||||
* Some participants reported more than one reason for withdrawing. |
|||||||||||||||
4 Adverse events reported by Australian soldiers in the first contingent* after taking mefloquine (n = 536) or doxycycline (n = 388) for malaria prophylaxis in East Timor, Apr–Oct 2001
|
Mild degree |
Moderate degree |
Severe degree |
||||||||||||
|
Mefloquine |
Doxycycline |
Mefloquine |
Doxycycline |
Mefloquine |
Doxycycline |
|||||||||
Sleep disturbance |
128 (24%) |
53 (14%) |
33 (6%) |
28 (7%) |
2 (< 1%) |
2 (< 1%) |
|||||||||
Headache |
53 (10%) |
51 (13%) |
17 (3%) |
14 (4%) |
1 (< 1%) |
4 (1%) |
|||||||||
Tiredness |
72 (13%) |
78 (20%) |
20 (4%) |
16 (4%) |
0 |
1 (< 1%) |
|||||||||
Nausea |
86 (16%) |
63 (16%) |
22 (4%) |
20 (5%) |
4 (1%) |
0 |
|||||||||
* Some participants reported more than one adverse event. |
|||||||||||||||
- Scott J Kitchener1
- Peter E Nasveld2
- Robin M Gregory3
- Michael D Edstein4
- 1 Centre for Military and Veterans’ Health, Mayne Medical School, Herston, QLD.
- 2 Army Malaria Institute, Enoggera, QLD.
The authors would like to thank Private Michael Reid, Lieutenant Kieran McCarthy, Captain Anne Jensen, Captain Leith Baird, Captain Tracey Carthew, Captain John Cunningham, Captain Damien Woods, Squadron Leader Karen Leshinskas, Major Brett Robertson and Lieutenant Colonel Bob Cooper for either their medical or technical support towards the study. The opinions expressed are those of the authors and do not necessarily reflect those of the Defence Health Service or any extant ADF policy. The study was wholly conducted using the resources and personnel of the ADF.
The authors were full-time employees of the Australian Army Malaria Institute at the time this research was carried out and have no other conflict of interest to declare. The Australian Defence Human Research Ethics Committee, in accordance with National Health and Medical Research Council guidelines, requires all clinical research to be submitted for peer review.
- 1. World Health Organization. International travel and health. Malaria. Geneva: WHO, 2002.
- 2. Centers for Disease Control and Prevention. Health information for international travel, 2001-2002. Altanta: US Department of Health and Human Services. 2004. Available at: http://www.cdc.gov/travel/malariadrugs.htm (accessed Jan 2005).
- 3. Kitchener SJ, Auliff AM, Rieckmann KH. Malaria in the Australian Defence Force during and after participation in the International Force in East Timor (INTERFET). Med J Aust 2000; 173: 583-585. <MJA full text>
- 4. Nasveld P, Brennan L, Edstein M, et al. A randomised double-blind comparative study to evaluate the safety, tolerability and effectiveness of tafenoquine and mefloquine for the prophylaxis of malaria in non-immune Australian soldiers [abstract]. Am J Trop Med Hyg 2002; 67(Suppl 1): 255.
- 5. Croft AM, Clayton TC, World MJ. Side effects of mefloquine prophylaxis for malaria: an independent randomized controlled trial. Trans R Soc Trop Med Hyg 1997; 91: 199-203.
- 6. Jaspers CA, Hopperus Buma AP, van Thiel PP, et al. Tolerance of mefloquine chemoprophylaxis in Dutch military personnel. Am J Trop Med Hyg 1996; 55: 230-234.
- 7. Ohrt C, Richie TL, Widjaja H, et al. Mefloquine compared with doxycycline for the prophylaxis of malaria in Indonesian soldiers. Ann Intern Med 1997; 126: 963-972.
- 8. Peragallo MS, Sabatinelli G, Sarnicola G. Compliance and tolerability of mefloquine and chloroquine plus proguanil for long-term malaria chemoprophylaxis in groups at particular risk (the military). Trans R Soc Trop Med Hyg 1999; 93: 73-77.
- 9. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol 1993; 44: 257-265.
- 10. Overbosch D, Schilthuis H, Bienzle U, et al. Atovaquone-proguanil versus mefloquine prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin Infect Dis 2001; 33: 1015-1021.
- 11. Phillips MA, Kass RB. User acceptability patterns for mefloquine and doxycycline malaria chemoprophylaxis. J Travel Med 1996; 3: 40-45.
- 12. Bragonier R, Reyburn H, Nasveld P, et al. Rainy-season prevalence of malaria in Bobonaro district, East Timor. Ann Trop Med Parasitol 2002; 96: 739-743.





Abstract
Objectives: To describe the tolerability of mefloquine in Australian soldiers for malaria prophylaxis, including a comparison with doxycycline.
Design: Open-label, prospective study and cross-sectional questionnaire and interview.
Setting and participants: Two contingents of Australian soldiers, each deployed to East Timor for peacekeeping duties over a 6-month period (April 2001–October 2001 and October 2001–May 2002).
Outcome measures: Withdrawals during the study; adverse events relating to mefloquine prophylaxis; willingness to use mefloquine again on deployment.
Results: Of 1157 soldiers starting on mefloquine, 75 (6.5%) withdrew because of adverse responses to the drug. There were three serious adverse events of a neuropsychiatric nature, possibly relating to mefloquine. Fifty-seven per cent of soldiers using mefloquine prophylaxis reported at least one adverse event, compared with 56% using doxycycline. The most commonly reported adverse effects of both drugs were sleep disturbance, headache, tiredness and nausea. Of the 968 soldiers still taking mefloquine at the end of their deployments, 94% indicated they would use mefloquine again. Of 388 soldiers taking doxycycline prophylaxis who were deployed with the first mefloquine study contingent, 89% indicated they would use doxycycline again.
Conclusions: Mefloquine was generally well tolerated by Australian soldiers and should continue to be used for those intolerant of doxycycline.