We report an outbreak of a “rash” syndrome in patients attending methadone clinics in New South Wales. It presents with a pruritic, exanthematous or purpuric rash involving the trunk, limbs, palms and soles, which develops over a week and proceeds in most patients to desquamation (mainly of palms and soles) persisting for 3–4 weeks. Mucosae are not involved, and patients are generally systemically well. To date, the rash has affected 22% of 316 patients attending one methadone clinic in western Sydney, as well as patients in clinics elsewhere in Sydney and rural NSW. The aetiology is as yet unknown.
A man aged in his 30s presented to a hospital emergency department with a 3-day history of a petechial and purpuric rash. He was an intravenous drug user who had been in a methadone treatment program for 7 years. He intermittently injected his oral methadone intravenously, most recently 24 hours before onset of the rash. This initially involved the lower limbs, but spread over 24 hours to affect the buttocks, lower back and abdomen. Associated but relatively mild symptoms included malaise and nausea for a week before rash onset, followed by sore throat, myalgia, ankle arthralgia, abdominal and chest pain and vomiting.
At presentation, the patient was afebrile. Blood pressure was 120/60 mmHg, and pulse 70 bpm. A sparse petechial and purpuric rash was present on lower limbs, feet, buttocks and lower abdomen. There was no pedal oedema, joint effusion or tenderness. There were no abnormalities on respiratory and cardiovascular examination, no clinical evidence of endocarditis, no lymphadenopathy, and mucosae were normal. The right upper abdominal quadrant was tender, but the liver and spleen were not enlarged, and no renal masses were palpable. Investigations were uninformative (Box 1).
Inpatient progress was unremarkable, and, 10 days after presentation, all symptoms had resolved, despite ongoing oral and intravenous methadone use.
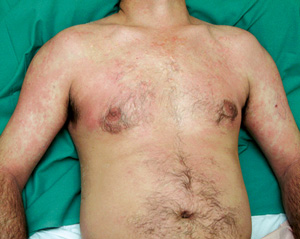
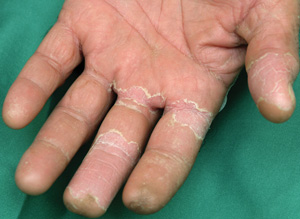
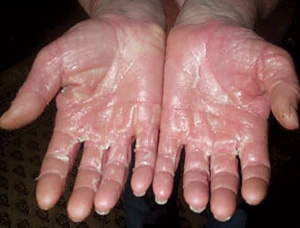
A middle-aged man presented with a 2-day history of an erythematous, pruritic rash over his trunk and limbs which was now beginning to desquamate. He was also an intravenous drug user in a methadone treatment program. In addition to taking prescribed oral methadone, he intermittently injected both methadone and stimulants, such as amphetamine, intravenously. There was no history of fever, oropharyngeal, genital, eye or systemic symptoms. On examination, he was afebrile, looked well and had a generalised exanthem, with erythema and significant desquamation of soles and palms (Box 2, A and B). There were no oral, mucosal or eye signs, and no lymphadenopathy or hepatosplenomegaly. Results of investigations were unremarkable (Box 1).
He was treated for 2 days with oral prednisolone and an antihistamine, and then discharged. The rash settled over a week, although he continued to have desquamation of the soles and palms 2 weeks later.
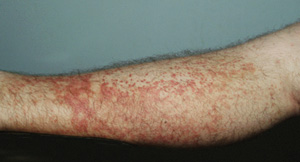
A young man who was an intravenous drug user in a methadone treatment program presented to the same hospital with a 2-day history of a purpuric lower-limb rash. In addition to taking prescribed oral methadone, he intermittently injected both heroin and methadone intravenously. Five days before presentation, he developed bilateral calf pain and generalised myalgia. He was initially seen at another hospital, where he was treated with broad-spectrum intravenous antibiotics for presumed sepsis. He discharged himself after 24 hours and was admitted to our hospital about 12 hours later because of his concern about the rash.
On admission, he was afebrile, with blood pressure of 115/65 mmHg and pulse of 75 bpm. He had a prominent purpuric rash involving both lower limbs (Box 2C), with sparse lesions on both forearms. Mucosae were normal, and there was no meningism, lymphadenopathy, hepatosplenomegaly, joint swelling or tenderness, no abnormalities on respiratory and cardiac examination, and no stigmata of endocarditis. Results of investigations were once again unremarkable (Box 1). The patient remained well despite the rash and was discharged from hospital 24 hours after admission.
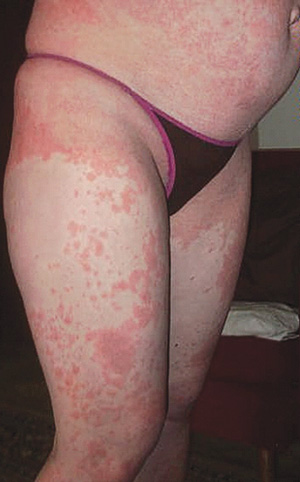
A young woman who was an intravenous drug user in a methadone treatment program presented to the methadone clinic with a 4-day history of an erythematous, pruritic rash over her trunk, limbs and hands. Other than oral methadone, she was taking no drugs and was otherwise well. Examination revealed an extensive exanthem over her trunk, hands and legs. She had no fever, and blood pressure was normal. She was reviewed a week later and still had an extensive generalised erythematous exanthem, as well as finger and palm desquamation (Box 2, D and E). Results of investigations were unremarkable (Box 1).
The aetiology of this “rash” syndrome is yet to be elucidated. Currently, it appears to be restricted to people using methadone syrup, with no reports of rash in over 100 patients in western Sydney prescribed buprenorphine for treatment of opioid dependence, nor among non-methadone-using family members of patients with the rash, nor among healthcare workers in contact with these patients. To date, all patients with the “rash” syndrome who have been assessed for hepatitis C exposure are seropositive, but not all are viraemic. Some patients with the “rash” syndrome smoke cannabis and intermittently inject methadone or other drugs. However, these characteristics are not universal among affected patients, nor more frequent than in unaffected patients on the methadone program, among whom they are also common. Similarly, the use of prescription or complementary medicines does not seem to be associated with the “rash” syndrome.
Similar rashes and associated desquamation are common in staphylococcal and streptococcal toxin-induced illnesses, such as toxic shock syndrome and scalded skin syndrome,1-3 and in some viral illnesses, such as parvovirus infection and measles.4 However, the patients in the current outbreak did not give a history of bacterial or viral illness, and family members not taking methadone do not appear to have developed the syndrome. HIV antibody testing has been performed in some affected patients and has been negative. Throat swabs taken in some patients have grown only normal respiratory flora. Markers of streptococcal infection, such as antideoxyribonuclease B antibodies and anti-streptolysin O titre, are positive in some but not all patients.
Skin biopsy performed in a number of patients has failed to help define the aetiology of the rash. Histological examination often shows focal and mild spongiosis with superficial perivascular chronic inflammation, while direct immunofluorescence examination shows deposition of IgM and complement 3 in dermal capillaries. These findings are consistent with an immunological reaction in the skin, but do not clarify whether it is the primary cause of the rash or a secondary phenomenon. A hypersensitivity reaction to a contaminant in the methadone syrup could present with such a picture.
The fact that, to date, all the patients identified in western Sydney had been taking methadone syrup from a single manufacturer raises the possibility of batch contamination; however, batches are distributed nationally, so more widespread involvement would probably be expected if this was the basis of the syndrome. In addition, examination of the methadone syrup has failed to detect any contamination. The possibility of alternative sources of contamination, such as methadone storage or delivery devices, remains to be explored.
We believe it is important for physicians to be aware of this newly emerging syndrome, both to assist with more accurate delineation of its epidemiology and pathogenesis, and to permit more effective investigation and treatment of affected patients. State public health units and the Therapeutic Goods Administration are investigating this outbreak to try to determine the cause of this new syndrome.
1 Results of investigations in four patients with rash
Investigations |
Reference range |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
||||||||||
Full blood count and film |
Normal; platelet aggregates on film |
Normal apart from WBC 10.8 x 109/L; occasional reactive lymphocytes |
Normal |
Normal, apart from Hb 107 g/L |
|||||||||||
Haemoglobin (Hb) (g/L) |
115–161 |
||||||||||||||
White blood cell count (WBC) (x 109/L) |
3.7–9.5 |
||||||||||||||
ESR (mm/h) |
0–15 |
4 |
11 |
5 |
38 |
||||||||||
C-reactive protein (mg/L) |
0–11 |
20 |
27 |
22 |
15 |
||||||||||
Liver function tests |
Normal |
Abnormal |
Abnormal |
Normal |
|||||||||||
γ-Glutamyltransferase (U/L) |
8–43 |
47 |
48 |
||||||||||||
Alanine aminotransferase (U/L) |
10–47 |
88 |
157 |
||||||||||||
Aspartate aminotransferase (U/L) |
12–45 |
104 |
154 |
||||||||||||
ANA, ANCA, ENAs, rheumatoid factor, complement C3 and C4 |
Normal |
nd |
Normal |
nd |
|||||||||||
Cryoglobulins |
Absent |
nd |
Detected |
nd |
|||||||||||
Prothrombin time (s) |
11–18 |
Normal |
Normal |
Normal |
nd |
||||||||||
APTT (s) |
25–36 |
Normal |
39 |
Normal |
nd |
||||||||||
Hepatitis C virus |
IgG-positive; undetectable viral load (< 600 IU/mL) |
IgG-positive; viral load not assessed |
IgG-positive; viral load > 850 000 IU/mL |
IgG-positive; refused viral load assay |
|||||||||||
HIV antibody |
Negative |
nd |
nd |
nd |
|||||||||||
Urinalysis |
Trace protein (39 mg/24 h); no casts/red cells |
Normal |
Normal |
nd |
|||||||||||
Blood culture |
Negative |
Negative |
Negative |
nd |
|||||||||||
Throat swab |
Nd |
Normal flora |
nd |
nd |
|||||||||||
Electrocardiogram |
Normal |
nd |
nd |
nd |
|||||||||||
Chest x-ray |
Normal |
Normal |
Normal |
nd |
|||||||||||
Echocardiogram |
Transthoracic normal; transoesophageal not tolerated by patient |
nd |
nd |
nd |
|||||||||||
ESR = erythrocyte sedimentation rate. nd = not done. ANA = antinuclear antibody. ANCA = antineutrophil cytoplasmic antibody. ENAs = extractable nuclear antigen antibodies. APTT = activated partial thromboplastin time. |
|||||||||||||||
- 1. Edlich RF, Horowitz JH, Nichter L, et al. Clinical syndromes caused by staphylococcal epidermolytic toxin. Compr Ther 1985; 11: 45-48.
- 2. Floret D. Clinical aspects of streptococcal and staphylococcal toxinic diseases. Arch Pediatr 2001; 4: 762s-768s.
- 3. Hoge CW, Schwartz B, Talkington DF, et al. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. A retrospective population-based study. JAMA 1993; 269: 384-389.
- 4. Amurao GV, Gottwald LD, Duggan J, et al. Vaccine era measles in an adult. Cutis 2000; 66: 337-340.





None identified.