Although often perceived as trivial or “inconvenient”, allergic rhinitis (AR) is a major chronic respiratory disease by virtue of its high prevalence and significant effect on quality of life, work or school performance, and productivity.1
The prevalence of all allergic diseases, including AR, has increased dramatically over the past few decades, particularly in developed countries. One study of self-reported asthma and hayfever in Tasmanian adults revealed the lifetime prevalence of hayfever among adults had doubled from 19.2% in 1968 to 41.3% in 1991–1993.2
Data collected in general practice suggest the national point prevalence of AR is 18.7%, with the highest prevalence among the 25–44-years age group (24.4%).3 Children also feature as sufferers, with the prevalence of current AR at 12% in 6–7-year-olds and 19.6% in 13–14-year-olds.4
For people with AR, quality of life can be considerably reduced, leading to impaired performance of daily activities, cognitive function and classroom productivity, and reduced psychosocial wellbeing.5 AR also creates a significant economic burden: the Australian Institute of Health and Welfare estimated that, in 1994, respiratory disease (including AR) accounted for 8.0% of total healthcare system costs.6
Poorly controlled AR may be associated with various complications and comorbid conditions. AR and asthma frequently co-exist, and AR usually precedes and is a significant risk factor for asthma.1,7-9 Furthermore, evidence shows that treating AR, particularly with intranasal corticosteroids (INCS), can reduce asthma-related emergency department visits and hospitalisations.10 Other conditions that are associated with AR include sinusitis,11 otitis media1,11 and nasal polyposis.1
There have been various international developments in the approach to managing AR. In addition, recent changes to the scheduling of drugs used in treating allergic disease in Australia have important repercussions for the management of this disease and, potentially, for the risk of complications. This article is intended to highlight these changes and address the changing treatment environment for AR in Australia.
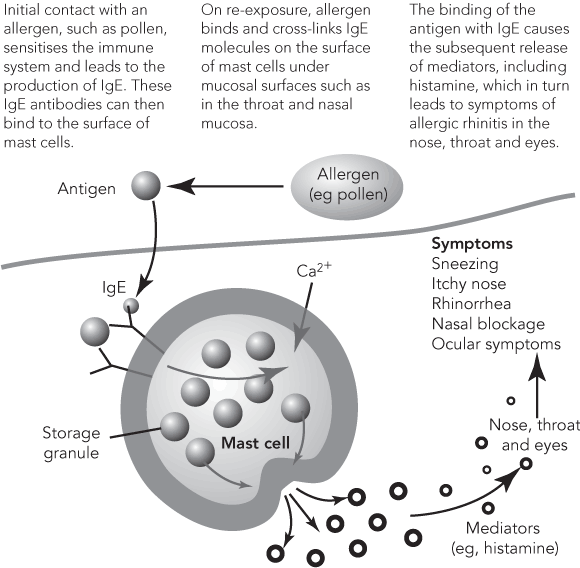
AR is a symptomatic disorder of the nose induced by inflammation mediated by immunoglobulin E (IgE) in the membrane lining the nose after allergen exposure.1 Allergic individuals become sensitised to and may develop IgE antibodies against allergens such as pollen, dust mites, animal dander and mould spores. Antigen-specific IgE binds to high-affinity receptors on tissue mast cells.12 On further exposure to the specific allergen, antigen binds to IgE antibodies on the mast cells and mast-cell degranulation occurs, leading to release of chemical mediators (Box 1).12
This immediate allergic response to antigen is termed the early-phase response. The mediators released during this phase include histamine, kinins, neutral proteases, cytokines (such as tumour necrosis factor α) and a variety of interleukins.12 Activation of mast cells also leads to the production of leukotrienes and prostaglandins from arachidonic acid. Together, these mediators result in the characteristic symptoms of AR — watery rhinorrhoea, sneezing and itching — within minutes of allergen exposure.12
This is followed several hours later by the late-phase response, involving the infiltration of inflammatory cells and the release of mediators into the nasal mucosa. The symptoms are essentially the same as in the early-phase response, but congestion predominates.
Diagnosis of AR requires a detailed and accurate history, physical examination, and allergy investigation to assist in characterising the patient and identifying the provoking factors.1 Obtaining a general medical history should be followed by questions on personal and family history of allergic conditions, including eczema, asthma and AR, as well as details of the patient’s environment and occupation. It is important to be mindful that, although the classical symptoms of itching, irritative nose, rhinorrhoea and eye symptoms particularly favour a diagnosis of AR, they are not specific, and may be the result of a viral infection or vasomotor rhinitis. Conversely, their absence does not exclude AR, as blockage may be the predominant symptom in persistent AR. The physical examination should focus on the nose, throat, eyes, ears and, when appropriate, lung function.1
Allergy testing should be undertaken to determine the presence of allergen-specific IgE. This can be done by skin-prick testing or radioallergosorbent test. Allergens are selected according to probability based on clinical history.1 Positive test results should be coupled with clinical features consistent with AR to affirm diagnosis. For more detail on the diagnostic process, readers are referred to a recent article by Puy.13
The original classification of AR was based on the timing of allergen exposure — seasonal or perennial. A newer system, proposed in the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines in 2001, classifies AR according to the timing of symptom presentation — intermittent or persistent (Box 2).1
The ARIA system is more appropriate for use in Australia for several reasons:
With the traditional system, “perennial” refers to AR caused by indoor allergens such as dust mites, moulds, insects and animal dander, whereas “seasonal” allergy is related to outdoor allergens such as pollens and moulds. However, in certain regions of Australia, pollens and moulds may be perennial allergens.
Even patients allergic to “seasonal” allergens may have symptoms for much of the year. For example, in certain regions of Australia, particularly Queensland, it can be very difficult to define the pollen season.
Most patients are sensitised to multiple allergens — many patients allergic to pollen are also allergic to moulds — meaning that symptoms may be present throughout the year.1
The goal of management is to achieve optimal symptom control. Achieving this goal depends on what triggers the condition, what the symptoms are, and symptom severity.
The first approach that should be considered is minimising exposure to environmental trigger factors. This approach is relatively harmless.14 Although two meta-analyses of trials of allergen avoidance through reduction of house dust mite antigen proved inconclusive,14,15 authors of both reviews called for further adequately powered trials to optimise clinical practice. Avoidance measures are biologically plausible, and many practitioners view them as valuable. Encouragingly for AR patients, the nose may be more responsive to avoidance measures than the lower airways.16
With a range of over-the-counter (OTC) treatments for AR available (Box 3), most Australian adults now self-medicate for AR. A 2002 survey of hayfever and allergy sufferers revealed that nearly two-thirds of respondents did not consult their doctor about their current AR treatment (Newspoll Omnibus. Data on file, GlaxoSmithKline). The current options for self-medication include antihistamines, INCS and decongestants.
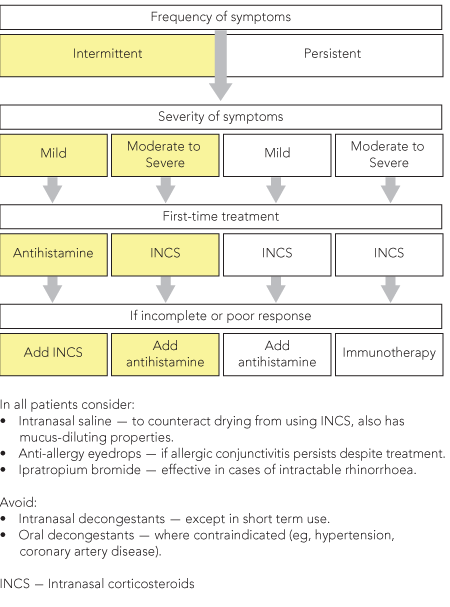
Of these options, INCS are the most effective and are considered first-line therapy for persistent AR and for moderate to severe intermittent AR in adults.1,17-20 In systematic reviews and meta-analyses, INCS have been shown to be superior to antihistamines in controlling nasal symptoms of AR.19-21 Moreover, INCS are more cost-effective than oral antihistamines.19
INCS are well tolerated and can be used long term without atrophy of the nasal mucosa.1,17 Most side effects are due to local irritation. Studies in adults suggest minimal side effects with use of nasal steroids in recommended doses. Furthermore, studies of new steroid preparations, even in relatively high doses, reveal no systemic steroid effect on the hypothalamic–pituitary–adrenal axis, as assessed by morning cortisol concentrations, cosyntropin stimulation and 24-hour urinary free cortisol excretion.18
There is no clear evidence that one preparation is more effective than another.22 The two areas in which a difference exists are in systemic bioavailability and cost. Bioavailability is the percentage of the drug systemically available for pharmacological action after topical administration. It reflects absorption across the nasal mucosa (greater for water-soluble drugs such as budesonide), absorption of swallowed drug, and the degree to which the drug is inactivated by first-pass metabolism in the liver.23
We suggest that, for adults who are not receiving other corticosteroids, the choice of INCS can be guided by personal preference, cost and accessibility. For children and all patients who are also receiving inhaled, topical or systemic preparations, a drug with low bioavailability (fluticasone propionate, mometasone furoate) should be chosen, and dose of INCS taken into account in assessing total corticosteroid administered.
Older antihistamines are still available and are used extensively in combination with oral decongestants for cold and flu remedies (Box 4). These earlier antihistamines commonly cause central nervous system (CNS) effects such as somnolence, sedation, drowsiness, fatigue, loss of attention and impaired psychomotor performance, as well as anticholinergic effects, including difficulty in micturition, impotence, constipation and other gastrointestinal symptoms.24 Because of the sedative effects, these drugs are contraindicated in patients who undertake activities such as driving. They are not the drugs of choice in children. The second-generation antihistamines have a more favourable side-effect profile.
Other effective therapies also need to be used with care. In cases of severe nasal blockage, intranasal decongestants can be used for 2–3 days to improve access to the nasal mucosa for INCS, but overuse of these can result in rhinitis medicamentosa.
Oral decongestants are helpful in specific cases (for example, if a patient needs to travel by plane). However, their use is contraindicated in some groups, including people with hypertension or coronary artery disease, and they should be used with caution in patients with heart disease, hyperthyroidism, elevated intraocular pressure, prostatic enlargement or bladder dysfunction. Common adverse effects involve CNS stimulation and include nervousness, excitability and insomnia.25
Anti-allergy eye drops are an effective adjunctive therapy when ocular symptoms persist despite treatment (Box 5). Mast-cell stabilisers and cromones are safe for medium- or long-term use, but require regular and frequent use for optimal results.
For patients whose symptoms are not adequately controlled by OTC therapies, prescription treatments are available, including certain INCS. Systemic steroids should only be used in exceptional circumstances in adults on a short-term basis.
New treatment options, such as leukotriene-receptor antagonists (eg, montelukast), are being evaluated for use in managing AR. However, the results to date suggest they are not more efficacious than INCS.26
Adherence to an evidence-based protocol for the treatment of seasonal AR, such as the one presented in this article, produces better outcomes for patients than unstructured application of various treatment modalities.27 Box 6 outlines the key decision-making steps in determining optimal pharmacotherapy for adults with AR.
The diagnosis and treatment of AR in children follows essentially the same process as in adults. However, selection of therapy in children requires careful consideration to balance the pros and cons of the treatment options (Box 7).
INCS are now the drugs of choice in children with AR.28 Concerns that INCS may cause systemic side effects, such as suppression of growth and bone metabolism, have been allayed.23 One study showed that fluticasone has no clinically significant effect on lower-leg growth velocity in children aged 4–11 years.30 Mometasone use shows no evidence of growth suppression in children aged 3–9 years,31 and a recent trial of budesonide in children aged 5–15 years revealed no negative effects on growth.32 Local side effects of INCS are also minimal. After 3 months’ use of fluticasone in children aged 3–11 years, rhinoscopy showed no evidence of thinning of the nasal tissues or atrophy of the nasal mucosa.33
Second-generation antihistamines are useful when mild or intermittent symptoms are present. Older compounds, such as chlorpheniramine and diphenhydramine, should be avoided, as they can cause CNS dysfunction, impair cognition and increase somnolence, thereby exacerbating the effects of AR on children’s learning.34,35
Although some drugs (eg, loratadine) are indicated for children from 1 year of age, children younger than 2 years should be referred to an allergist/immunologist for diagnosis and subsequent management.
The rescheduling to Schedule 2 of beclomethasone in 2003 and budesonide, mometasone and fluticasone in 2004 gives patients access to a wider range of effective treatments. With these changes in scheduling comes a shift in the burden of care. The pharmacy will become the focal point for initiation of treatment for many patients. Educational programs are essential to ensure that pharmacists and their assistants are appropriately equipped to counsel patients with allergic disease.
However, there is a continued need for a team approach. When faced with a patient whose condition is unresponsive to OTC treatments, it is important for pharmacists to advise patients to speak to their general practitioner (Box 8). Similarly, GPs can be confident that referral for specialist review of patients with seemingly intractable, chronic or complex disease will provide access to effective alternative treatment options, such as immunotherapy.
The beneficial effects of allergen-specific immunotherapy on symptoms and quality of life in patients with AR are now widely accepted.18 Patients with AR should be considered candidates for immunotherapy based on severity of symptoms, failure of previous treatment strategies, the desire to avoid reliance on pharmacotherapy, and presence of comorbid conditions.18 Box 9 outlines the criteria for selecting candidates for immunotherapy.36 To avoid serious harm, immunotherapy must be prescribed and administered in accordance with the Australian guidelines for asthma.36 That is, it should be prescribed only by practitioners or teams with appropriate expertise, and administered by clinicians with training and experience in this form of treatment, in a setting with adequate resuscitation facilities and equipment.36
AR is not a “trivial” or “nuisance” condition; it can have significant detrimental effects on psychological wellbeing and school or work performance. Treatment can provide optimal symptom control and greatly improve quality of life. Moreover, treatment reduces the potential for more severe comorbidity, such as asthma.
Various treatment options are available, from simple strategies for allergen avoidance, to pharmacotherapies (prescription and OTC) and immunotherapy. Treatment choice depends on the individual’s symptoms and response to previous therapies. At least two out of three patients with AR select OTC medication (Newspoll Omnibus, 2002. Data on file, GlaxoSmithKline), with INCS the first-line choice.
2 ARIA guidelines for classification of allergic rhinitis1
Timing of symptoms |
|||||||||||||||
Intermittent |
Symptoms present for:
|
||||||||||||||
Persistent |
Symptoms present for:
|
||||||||||||||
Severity and quality of life |
|||||||||||||||
Mild |
|
||||||||||||||
Moderate to severe |
One or more of the following are present:
|
||||||||||||||
ARIA = Allergic Rhinitis and its Impact on Asthma. Adapted with permission from Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001; 108: S147-S334. Copyright 2001, American Academy of Allergy, Asthma and Immunology. |
|||||||||||||||
3 Treatment options for allergic rhinitis in adults and children aged 12 years or older (as at August 2004)
Schedule 2 (Pharmacy Medicine) |
|
Intranasal corticosteroids |
|
Beclomethasone dipropionate 50 g/spray |
2 sprays into each nostril, twice daily (maintenance: 1 spray into each nostril, once daily) |
Budesonide 32 g/spray |
4 sprays into each nostril, once daily, morning, or 2 sprays into each nostril twice daily (maintenance: 2 sprays into each nostril once daily, morning, or 1 spray into each nostril twice daily) |
Fluticasone propionate 50 g/spray |
2 sprays into each nostril, once daily, morning (maintenance: 1 spray into each nostril, once daily) |
Antihistamines (see also Box 4) |
|
Azelastine hydrochloride 0.14 mL/spray |
1 spray into each nostril, twice daily |
Cetirizine HCl 10 mg/tablet (for use in adults) |
1–2 tablets once daily |
Fexofenadine HCl 60, 120, or 180 mg/tablet |
1 tablet (60 mg), twice daily or 1 tablet (120 or 180 mg) once daily |
Fexofenadine HCl 60 mg + pseudoephedrine HCl 120 mg/tablet |
1 tablet every 12 hours |
Loratadine 10 mg/tablet |
1 tablet, once daily |
Loratadine 10 mg + pseudoephedrine sulfate 240 mg/tablet |
1 tablet, once daily |
Levocabastine HCl 0.5 mg/ml |
2 sprays in each nostril, twice daily |
Intranasal decongestants |
|
Oxymetazoline HCl 500 g/ml |
1–3 sprays in each nostril, twice daily |
Xylometazoline HCl 0.1 mg/ml |
1 spray in each nostril, up to 4 times daily |
Oral decongestants |
|
Phenylephrine HCl 5 mg/5 ml |
10 mL every 4 hours |
Intranasal mast-cell stabilisers |
|
Sodium cromoglycate 2.6 mg/spray |
1 spray into each nostril 4 times daily |
Intranasal anticholinergics |
|
Ipratropium bromide 21 or 42 g/15 mL |
2–4 sprays (21 g) into each nostril 2–3 times daily, or 1–2 sprays (42 g) 2–3 times daily |
Schedule 3 (Pharmacist Only Medicine) |
|
Antihistamines (See Box 4) |
|
Oral decongestants |
|
Pseudoephedrine HCl 60 mg/tablet |
1 tablet 3–4 times daily |
Schedule 4 (Prescription Only Medicine) |
|
Intranasal corticosteroids |
|
Budesonide 64 g/spray |
2 sprays in each nostril, once daily (morning) or 1 spray in each nostril twice daily (maintenance: 1 spray in each nostril, once daily) |
Mometasone furoate 50 g/spray |
2 sprays in each nostril, once daily (maintenance: 1 spray in each nostril, once daily) |
4 Over-the-counter first-generation antihistamines
Schedule 2
Brompheniramine maleate + phenylephrine HCl
Brompheniramine maleate + dextromethorphan hydrobromide + phenylephrine HCl
Chlorpheniramine maleate + paracetamol + pseudoephedrine HCl
Chlorpheniramine maleate + phenylephrine HCl
Diphenhydramine HCl + ammonium chloride + sodium citrate
Triprolidine HCl + pseudoephedrine HCl
Triprolidine HCl + paracetamol + pseudoephedrine HCl
Schedule 3
Azatadine maleate
Cyproheptadine HCl
Dexchlorpheniramine maleate
Diphenhydramine HCl + phenylephrine HCl
Methdilazine HCl
Pheniramine maleate
Promethazine HCl
5 Ocular treatments
Ocular lubricants
Various
Antihistamines
Anatazoline
Levocabastine
Antihistamines with vasoconstrictors
Naphazoline HCl + pheniramine maleate
Mast-cell stabilisers
Iodoxamide trometarol
Olopatadine HCl
Cromones
Sodium cromoglycate
7 Pros and cons of pharmacotherapeutic options for allergic rhinitis in children
Pros |
Cons |
||||||||||||||
Antihistamines |
|
||||||||||||||
|
|
||||||||||||||
Intranasal corticosteroids |
|
||||||||||||||
|
|
||||||||||||||
Decongestants |
|
||||||||||||||
|
|
||||||||||||||
8 Allergic rhinitis referral algorithm
Pharmacy-level referral criteria
Refer your patient to a GP if:
treatment with an antihistamine and an intranasal corticosteroid (INCS) has not controlled symptoms
symptom control requires long-term (> 1 month) treatment
the patient has comorbidities
the patient’s quality of life is being seriously affected
there are complications such as pain, anosmia or hearing loss.
General-practice-level referral criteria
Refer your patient to an allergy specialist if:
the patient requests it or wishes to identify triggers or obtain further information on the role of allergy in allergic rhinitis, and the GP considers it appropriate
the patient has severe allergic comorbidities such as eczema and food allergies, or troublesome or poorly controlled asthma
complications such as resistant obstruction, anosmia, sinus disease, ear problems, persistent purulent drainage, or behavioural effects are present
symptoms are persistent and/or severe and/or unresponsive
expensive or significant life-changing measures (eg, changing address) are being contemplated.
Refer your patient to an ear, nose and throat specialist if:
the patient has constant unilateral obstruction
complications such as resistant obstruction, anosmia, sinus disease, ear problems, persistent purulent discharge are present
a polyp is unresponsive to initial INCS therapy.
9 Criteria for immunotherapy in allergic rhinitis*18,36
Indications
A history indicating that exposure to a particular allergen precipitates symptoms and contributes to illness
Documented sensitivity to the clinically relevant aeroallergen
Future exposure to the allergen is unavoidable or only partially reducible
An effective allergen extract is available
Poor response to previous pharmacotherapy for allergic rhinitis
Patient-centred factors
Ability to give informed consent
Ability to commit to time for immunotherapy
Contraindications
Unstable asthma symptoms
Concomitant illness — immunotherapy is contraindicated in severe pulmonary and cardiovascular disease and should not be initiated in patients with autoimmune disease or malignancy
Pregnancy — initiation of immunotherapy during pregnancy is not recommended because of the risk to the fetus of a systemic allergic reaction
Taking β-blockers
* Medical practitioners who administer or supervise allergen immunotherapy must be familiar with the Australian guidelines regarding immunotherapy for asthma, as these guidelines are also applicable to allergic rhinitis.36
- Ronald S Walls1
- Robert J Heddle2
- Mimi L K Tang3
- Ben J Basger4
- Graham O Solley5
- Guan T Yeo6
- 1 Department of Immunology and Allergy, Concord Hospital, Concord, NSW.
- 2 Flinders Medical Centre, Bedford Park, SA.
- 3 Department of Immunology, Royal Children’s Hospital, Parkville, VIC.
- 4 Basger’s Pharmacy, North Bondi, NSW.
- 5 Watkins Medical Centre, Brisbane, QLD.
- 6 Berowra Family Medical Practice, Sydney, NSW.
This study was funded by an unrestricted educational grant from GlaxoSmithKline (GSK). The authors served as consultants on an advisory board for GSK, for which they received honoraria. GSK was not involved in the preparation of the manuscript, and approval was not sought from GSK for any of the material presented.
Assistance in preparation of the manuscript was provided by medical writers and designers employed by MediTech Media Asia Pacific Pty Ltd, who received financial support from GSK. GSK had no involvement in reviewing or approving the manuscript or its drafts.
- 1. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001; 108: S147-S334.
- 2. Hopper JL, Jenkins MA, Carlin JB, Giles GG. Increase in the self-reported prevalence of asthma and hay fever in adults over the last generation: a matched parent-offspring study. Aust J Public Health 1995; 19: 120-124.
- 3. Australian Institute of Health and Welfare GP Statistics and Classification Unit. SAND abstract No.1 from the BEACH program: Allergic rhinitis. Sydney: GPSCU University of Sydney; 2000. Available at: www.fmrc.org.au/allergicrhinitis.pdf (accessed Feb 2004).
- 4. Robertson CF, Dalton MF, Peat JK, et al. Asthma and other atopic diseases in Australian children. Australian arm of the International Study of Asthma and Allergy in Childhood. Med J Aust 1998; 168: 434-438. <MJA full text>
- 5. Fineman SM. The burden of allergic rhinitis: beyond dollars and cents. Ann Allergy Asthma Immunol 2002; 88: 2-7.
- 6. Australian Institute of Health and Welfare. Health expenditure Australia 2001–02. Health and Welfare Expenditure Series no. 17. Canberra: AIHW; 2003. (AIHW Catalogue no. HWE 24.)
- 7. Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol 2002; 109: 419-425.
- 8. Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc 1994; 15: 21-25.
- 9. Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol 2000; 106(5 Suppl): 201-205.
- 10. Fuhlbrigge AL, Adams RJ. The effect of treatment of allergic rhinitis on asthma morbidity, including emergency department visits. Curr Opin Allergy Clin Immunol 2003; 3: 29-32.
- 11. Mucha SM, Baroody FM. Relationships between atopy and bacterial infections. Curr Allergy Asthma Rep 2003; 3: 232-237.
- 12. Naclerio R. Clinical manifestations of the release of histamine and other inflammatory mediators. Allergy Clin Immunol 1999; 103: 382-385.
- 13. Puy R. Diagnosing and treating allergic rhinitis. Med Today 2003; 4: 14-22.
- 14. Sheikh A, Hurwitz B. House dust mite avoidance measures for perennial allergic rhinitis: a systematic review of efficacy. Br J Gen Pract 2003; 53: 318-322.
- 15. Gotzsche PC, Hammarquist C, Burr M. House dust mite control measures in the management of asthma: meta-analysis. BMJ 1998; 317: 1105-1110.
- 16. Rijssenbeek-Nouwens LH, Oosting AJ, Bruin-Weller MS, et al. Clinical evaluation of the effect of anti-allergic mattress covers in patients with moderate to severe asthma and house dust mite allergy: a randomised double blind placebo controlled study. Thorax 2002; 57: 784-790.
- 17. van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. Allergy 2000; 55: 116-134.
- 18. Dykewicz MS, Fineman S. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol 1998; 81: 478-518.
- 19. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ 1998; 317: 1624-1629.
- 20. Yanez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2002; 89: 479-484.
- 21. Stempel DA, Thomas M. Treatment of allergic rhinitis: an evidence-based evaluation of nasal corticosteroids versus nonsedating antihistamines. Am J Manag Care 1998; 4: 89-96.
- 22. Waddell AN, Patel SK, Toma AG, Maw AR. Intranasal steroid sprays in the treatment of rhinitis: is one better than the other? J Laryngol Otol 2003; 117: 843-845.
- 23. Allen DB. Systemic effects of intranasal steroids: an endocrinologist’s perspective. J Allergy Clin Immunol 2000; 106 Suppl 4: 179-190.
- 24. Simons FE. Non-cardiac adverse effects of antihistamines (H1-receptor antagonists). Clin Exp Allergy 1999; 29 Suppl 3: 125-132.
- 25. Australian Medicines Handbook. 5th ed. Adelaide: Australian Medicines Handbook Ltd, 2004.
- 26. Pullerits T, Praks L, Ristioja V, Lotvall J. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol 2002; 109: 949-955.
- 27. Bousquet J, Lund VJ, Van Cauwenberge P, et al. Implementation of guidelines for seasonal allergic rhinitis: a randomized controlled trial. Allergy 2003; 58: 733-741.
- 28. Galant SP, Wilkinson R. Clinical prescribing of allergic rhinitis medications in the preschool and young school-age child: what are the options? BioDrugs 2001; 15: 453-463.
- 29. Scadding GK. Corticosteroids in the treatment of pediatric allergic rhinitis. J Allergy Clin Immunol 2001; 108: S59-S64.
- 30. Skoner DP, Gentile D, Angelini B, et al. The effects of intranasal triamcinolone acetonide and intranasal fluticasone propionate on short-term bone growth and HPA axis in children with allergic rhinitis. Ann Allergy Asthma Immunol 2003; 90: 56-62.
- 31. Schenkel EJ, Skoner DP, Bronsky EA, et al. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics 2000; 105: e22.
- 32. Moller C, Ahlstrom H, Henricson KA, et al. Safety of nasal budesonide in the long-term treatment of children with perennial rhinitis. Clin Exp Allergy 2003; 33: 816-822.
- 33. Richards DH, Milton CM. Fluticasone propionate aqueous nasal spray: a well-tolerated and effective treatment for children with perennial rhinitis. Pediatr Allergy Immunol 1996; 7: 35-43.
- 34. Simons FE, Fraser TG, Reggin JD, et al. Adverse central nervous system effects of older antihistamines in children. Pediatr Allergy Immunol 1996; 7: 22-27.
- 35. Simons FE, Reggin JD, Roberts JR, Simons KJ. Benefit/risk ratio of the antihistamines (H1-receptor antagonists) terfenadine and chlorpheniramine in children. J Pediatr 1994; 124: 979-983.
- 36. Thoracic Society of Australia and New Zealand and the Australasian Society of Clinical Immunology and Allergy. Specific allergen immunotherapy for asthma. Med J Aust 1997; 167: 540-544. <MJA full text>





Abstract
Allergic rhinitis (AR) is one of the most prevalent medical conditions. It has significant effects on quality of life and can have considerable socioeconomic effects.
The traditional classification of perennial and seasonal rhinitis does not distinguish between provoking factors, nor does it indicate the most appropriate treatment. A more useful classification is based on symptoms, which may be intermittent or persistent, and vary widely in severity.
The goal of management is to achieve optimal symptom control. Therapeutic options include allergen avoidance, pharmacotherapy and immunotherapy.
Antihistamines and intranasal corticosteroids (INCS) have become the cornerstones of therapy. A variety of effective treatments are available for consumers to self-select, without the advice of a doctor or pharmacist.
INCS are widely recognised as the most effective pharmacotherapy for AR, in both adults and children. The efficacy of various preparations is similar, but those with low systemic bioavailability are preferred for children and for patients who are also receiving inhaled, topical or systemic corticosteroids.