The Australian Pharmaceutical Benefits Scheme (PBS), which subsidises the prescription of essential marketed drugs, currently covers nearly 600 generic drugs in over 2500 products. It processes 158 million prescriptions annually, representing about 80% of all prescriptions written, costing over $4.5 billion annually and this cost is rising at about 7% per annum.1 By reducing patients’ out-of-pocket costs, the PBS provides a strong incentive towards prescribing within this scheme, and, for many drug classes, PBS data reflect a large proportion of all filled prescriptions for ambulatory patients. Thus, aggregate PBS prescription data can provide a comprehensive picture of androgen prescribing, excluding only in-hospital and private prescriptions. To date, no analyses of androgen prescribing patterns have been reported in this country. We examined temporal trends and geographical patterns of androgen prescribing in Australia over the past 11 years.
Over 1991–2001, there was a minimal increase in net TE prescribing, consistent with population growth (Box 1). There were two upsurges of prescribing — 1993 to 1994, and 1998 to 1999. Each upsurge waned during periods that corresponded to changes in access to PBS prescribing.
TU prescribing over 1991–2001 changed in parallel with changes in TE, but the changes were more extreme (Box 1). There was a fourfold increase from 1991 to 1994, followed by a sharp decline of nearly 50% around 1994, when an authority requirement for androgen prescribing was introduced. After the mid-1990s, there was a gradual rise, which was stemmed by the introduction of PBS restrictions in 2000, based on Endocrine Society of Australia consensus guidelines.2
There were consistent but stable differences between states and territories for prescribing of TE and TU (Box 2). The national trends, with the two prescribing peaks described above, were mirrored to some degree in each state, except for Western Australia. There, a steep rise to fourfold above the national average in prescribing of TU and TI (but not TE) was observed, coinciding with the opening of a franchised “andropause” clinic in 1996, linked to a GP whose standard practice highlighted prescribing of TU and TI but not TE (Box 3).
To determine whether the upsurge in testosterone prescribing in Western Australia might reflect improved diagnosis of genuine androgen deficiency, a surrogate marker for new diagnosis of androgen deficiency in younger men was examined. As karyotyping is the sine qua non for the diagnosis of Klinefelter’s syndrome,3 the number of Medicare reimbursements for cytogenetic tests was examined. On a per-capita basis, the number of Medicare reimbursements for cytogenetic tests performed in Western Australia did not deviate significantly from the national average over 1994 to 2002 (data not shown).
During the 1990s, there were no significant changes in the indications for testosterone treatment4 and, in particular, no convincing evidence of any quality-of-life benefit to justify testosterone therapy for older men without overt androgen deficiency.5,6 Yet, despite this, there was a 20-fold increase in revenue from testosterone sales in the United States,7 driven by dramatic increases in popular, professional and commercial interest in androgen therapy for ageing men. Our study shows that, over 1991 to 2001, testosterone prescribing in Australia increased modestly overall, far less dramatically than in the US, and with significant differences in time trends, according to specific products, and by region. The two product classes highlighted in this study differ in that oral TU is more acceptable to middle-aged and older men than injections (the standard treatment), but daily treatment costs are about four times higher. Because of its pharmacological limitations (notably low bioavailability,8 meal-dependent oral bioavailability9 and erratic pharmacokinetics10), TU has remained a second-line testosterone product.
The two peaks and troughs in PBS prescribing of androgens during the decade coincide with significant changes in PBS prescribing regulations. The first surge, in the early 1990s, was most striking for TU, and this probably reflects marketing for this product. The parallel but smaller increase in TE prescribing may reflect increased general awareness of androgen deficiency resulting from the marketing campaign for TU. This early surge appeared to be partly reversed by the 1994 change to “authority required” status for androgens. This change, requiring a phone call to authorise each prescription, may have deterred poorly justified prescribing. The second peak late in the decade appears to reflect global effects of the marketing of transdermal androgens, and especially the first testosterone gel in the US in 1996, with the target being primarily older men (andropause: “male menopause”). This surge in PBS prescribing appears to have been blunted by the introduction of the specific PBS restrictions for androgen prescribing, which targeted androgen prescribing for older men without changing standard medical treatment for men with classical androgen deficiency because of underlying testicular or pituitary disease. However, the PBS restriction may have encouraged more private (non-PBS) prescriptions for testosterone, despite the significant financial disincentive to patients.
Increased testosterone prescribing may reflect a response to genuine clinical need, to promotional activity, or a mixture of both. There is persuasive evidence that genuine androgen deficiency resulting from pituitary or testicular disease is underdiagnosed in the community. For example, in Denmark, less than 20% of men with Klinefelter’s syndrome are diagnosed during life,11 a finding supported by regular observations of new diagnoses of this congenital condition among older men, as well as corroborative evidence of underdiagnosis from United Kingdom data.12 As the finding of small firm testes in Klinefelter’s syndrome is virtually pathognomonic, this degree of underdiagnosis suggests many men never undergo full genital examination. As Klinefelter’s syndrome is the most readily identified clinical cause of hypogonadism, it is likely that other, less easily identified conditions are also underdiagnosed. Hence, the steep increase in androgen prescribing in Western Australia in the late 1990s might be the result of improved diagnosis of genuine androgen deficiency, for which the diagnosis of Klinefelter’s syndrome can be considered a surrogate marker. However, this is unlikely, as there was no corresponding increase in cytogenetic testing, the diagnostic test for Klinefelter’s syndrome. Another reason to refute this explanation is that, during the 1990s, standard initial treatment for men with newly diagnosed androgen deficiency was injectable testosterone esters before considering maintenance therapy with injectable, implantable or oral testosterone. Hence, the increase in prescribing of testosterone undecanoate and implants, but not injectable testosterone esters, is not consistent with increasing new diagnoses of Klinefelter’s syndrome. These patterns do correspond to the prescribing preferences and the temporal opening of a franchised “men’s health” clinic with a focus on treating “andropause” in Western Australia.
Our study highlights the irony that significant under-recognition of genuine androgen deficiency in younger men coexists with growing overuse of unproven testosterone treatment for older men. Our findings indicate that androgen prescribing for both valid and unproven indications is sensitive to community, commercial, professional and regulatory pressures, which act to promote or deter testosterone prescribing. Well targeted regulatory policy with complementary professional and community education is needed to correct underdiagnosis of genuine androgen deficiency and the potential overprescribing of testosterone as an unproven anti-ageing “tonic”. While pharmaceutical companies are accountable within advertising and industry professional codes, specialised clinics are free of such constraints. Effective professional and community education is needed to reinforce evidence-based medical practice while countering unbalanced direct marketing to the public.13
There are significant regional differences in elective surgery, including hysterectomy and laminectomy,14-18 as well as in prescriptions for oestrogen replacement therapy for menopausal women.19-22 Potential determinants of such differences include heterogeneity in medical beliefs, practice styles, referral patterns and availability of alternative treatments.22,23 Similar considerations apply to testosterone prescribing for ageing men, but few studies have analysed the influences on doctors’ prescribing patterns and behaviours. Our study shows that Australian PBS data provide an opportunity for such research.
1 National prescriptions for injectable testosterone esters and oral testosterone undecanoate between 1 January 1991 and 30 December 2001, based on aggregate Pharmaceutical Benefits Scheme (PBS) data
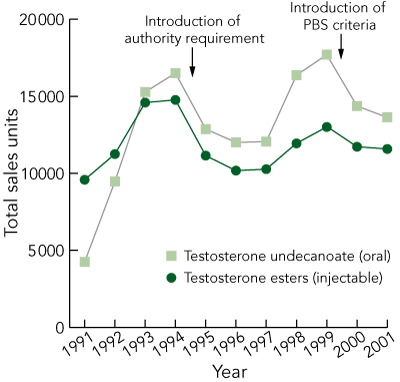
Note that plotted units reflect absolute national sales of a group of products and cannot be compared for different product classes in terms of daily dosage units.
2 Prescriptions for injectable testosterone esters and oral testosterone undecanoate between 1 January 1991 and 30 December 2001 for Australian states and territories, based on aggregate Pharmaceutical Benefits Scheme (PBS) data
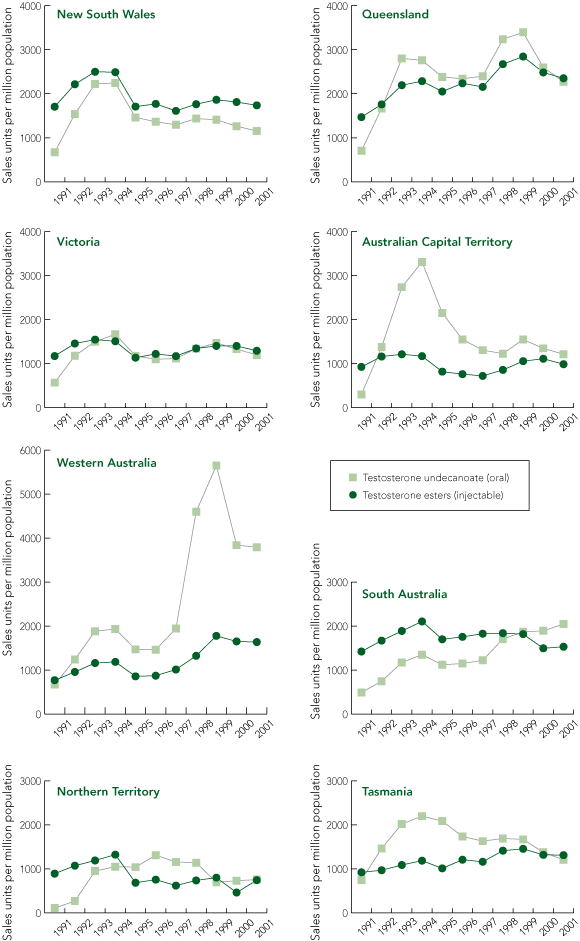
Note: Plotted units reflect sales of a group of products per million population and cannot be compared for different product classes in terms of daily dosage units.
3 Prescriptions for oral testosterone undecanoate, injectable testosterone esters and testosterone implants between 1 January 1991 and 30 December 2001 for Western Australia compared with the national average, based on aggregate Pharmaceutical Benefits Scheme (PBS) data
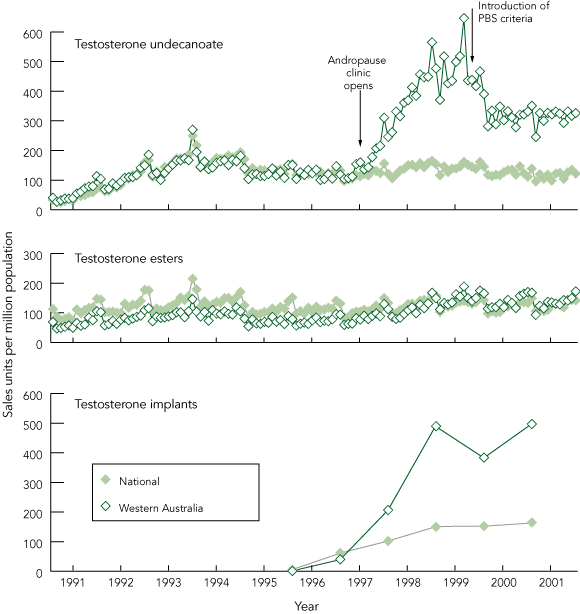
Note: Plotted units reflect sales of products per million of population and cannot be compared for different product classes in terms of daily dosage units.
Received 23 February 2004, accepted 22 April 2004
- David J Handelsman1
- ANZAC Research Institute, Concord Hospital, University of Sydney, Sydney, NSW.
The author thanks Paula Anderson for technical assistance, and Dr B Oddens (Organon Australia) for helpful comments and review of the manuscript. Funding support was received from Andrology Australia, the federally funded Australian Centre of Excellence in Male Reproductive Health.
The author has received institutional funding to design and perform clinical studies involving testosterone or other forms of androgen therapy from the World Health Organization, National Health and Medical Research Council, World Antidoping Agency and all pharmaceutical companies marketing androgens and related products in Australia (Organon, Schering, Serono, Pharmacia-Upjohn, Besins, SciGen, Lawley).
- 1. Australian Government Department of Health and Ageing. General Public. Pharmaceutical Benefits Scheme. About the PBS. Available at: www.health.gov.au/pbs/general/aboutus.htm (accessed Aug 2004).
- 2. Conway AJ, Handelsman DJ, Lording DW, et al. Use, misuse and abuse of androgens. The Endocrine Society of Australia consensus guidelines for androgen prescribing. Med J Aust 2000; 172: 220-224. <MJA full text>
- 3. Smyth CM, Bremner WJ. Klinefelter syndrome. Arch Intern Med 1998; 158: 1309-1314.
- 4. Handelsman DJ. Androgen action and pharmacologic uses. In: DeGroot LJ, editor. Endocrinology. 4th ed. Philadelphia: W B Saunders, 2001: 2232-2242.
- 5. Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc 2003; 51: 101-115.
- 6. Liverman CT, Blazer DG, editors. Testosterone and aging: clinical research directions. Washington, DC: Institute of Medicine, The National Academies Press, 2004.
- 7. Bhasin S, Singh AB, Mac RP, et al. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl 2003; 24: 299-311.
- 8. Shackleford DM, Faassen WA, Houwing N, et al. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J Pharmacol Exp Ther 2003; 306: 925-933.
- 9. Bagchus WM, Hust R, Maris F, et al. Important effect of food on the bioavailability of oral testosterone undecanoate. Pharmacotherapy 2003; 23: 319-325.
- 10. Conway AJ, Boylan LM, Howe C, et al. A randomised clinical trial of testosterone replacement therapy in hypogonadal men. Int J Androl 1988; 11: 247-264.
- 11. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 2003; 88: 622-626.
- 12. Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn 1997; 17: 363-368.
- 13. Handelsman DJ, Zajac JD. Androgen deficiency and replacement therapy in men. Med J Aust 2004; 180: 529-535. <MJA full text>
- 14. Easterday CL, Grimes DA, Riggs JA. Hysterectomy in the United States. Obstet Gynecol 1983; 62: 203-212.
- 15. Coulter A, McPherson K, Vessey M. Do British women undergo too many or too few hysterectomies? Soc Sci Med 1988; 27: 987-994.
- 16. MacLennan AH, MacLennan A, Wilson D. The prevalence of hysterectomy in South Australia. Med J Aust 1993; 158: 807-809.
- 17. Cherkin DC, Deyo RA, Loeser JD, et al. An international comparison of back surgery rates. Spine 1994; 19: 1201-1206.
- 18. Taylor R, Rushworth RL. Hysterectomy fractions in New South Wales, 1971-2006. Aust N Z J Public Health 1998; 22: 759-764.
- 19. Oddens BJ, Boulet MJ, Lehert P, et al. A study on the use of medication for climacteric complaints in western Europe–II. Maturitas 1994; 19: 1-12.
- 20. Boulet MJ, Oddens BJ, Lehert P, et al. Climacteric and menopause in seven South-east Asian countries. Maturitas 1994; 19: 157-176.
- 21. Jolleys JV, Olesen F. A comparative study of prescribing of hormone replacement therapy in USA and Europe. Maturitas 1996; 23: 47-53.
- 22. MacLennan AH, Wilson DH, Taylor AW. Hormone replacement therapy: prevalence, compliance and the “healthy women” notion. Climacteric 1998; 1: 42-49.
- 23. Vessey MP, Villard-Mackintosh L, McPherson K, et al. The epidemiology of hysterectomy: findings in a large cohort study. Br J Obstet Gynaecol 1992; 99: 402-407.p





Abstract
Objectives: To analyse temporal trends and geographical variations in testosterone prescribing in Australia.
Design and setting: An analysis of testosterone prescribing over the past 11 years according to products and region, determined by Pharmaceutical Benefits Scheme (PBS) expenditure in Australian states and territories.
Main outcome measure: Patterns of monthly PBS expenditure on injectable, oral and implantable testosterone products from 1 January 1991 to 30 December 2001, classified by state or territory.
Results: There were two periods (1993–1994 and 1998–1999) of striking upsurge followed by declines in national total prescribing of testosterone. These changes were more prominent for oral than injectable testosterone products, and patterns were similar in all regions, apart from a disproportionately higher peak in Western Australia in 1998. On a per-capita basis, Western Australia showed a dramatic increase in prescribing of oral and implantable, but not injectable, testosterone coinciding with the opening of a franchised men’s sexual health clinic in Perth.
Conclusion: The two striking upsurges in testosterone prescribing despite no convincing new evidence to justify them appear to reflect promotional activity to prescribe testosterone for older men, rather than overcoming the underdiagnosis of androgen deficiency related to pituitary or testicular disease in younger men. The curtailments after the introduced restrictions to PBS prescribing for older men without overt androgen deficiency were partial and temporary, suggesting that such regulatory barriers are only partly successful in counteracting the commercial and populist pressure driving excessive testosterone prescribing. Professional and community education is needed for appropriate diagnosis of genuine androgen deficiency in younger men, while discouraging unproven testosterone treatment for ageing men.