Interventions to achieve cessation of tobacco smoking have consistently been shown to be effective and cost-effective.1-3 There is a compelling body of evidence from Australia and other countries that expenditure on tobacco control programs, particularly anti-smoking advertising, is correlated with reduced smoking rates.1 In turn, reductions in tobacco use have been clearly shown to reduce healthcare costs. Applied Economics has recently estimated that, since 1975, $2 in Australian healthcare expenditure has been saved for every $1 spent on tobacco control campaigns.4 Wider benefits to the community of reduced tobacco use were estimated to have totalled $8.602 billion, so that the net present value (NPV) of tobacco control programs was estimated to be $8.427 billion, with a benefit to cost ratio of 50 : 1.
About 20% of the Australian population smoke tobacco, and smoking rates exceed 30% in 20–29-year-old men, and 25% in 20–29-year-old women.5 Despite the substantial evidence that effective tobacco control programs would result in long-term cost savings, such interventions have not been fully or optimally implemented in Australia. For example, the very successful 1997 federal government-funded mass media advertising campaign has not continued, and the federal government now funds only a couple of weeks of anti-smoking advertising each year.1 Most smokers cannot recall their GP advising them to quit smoking,6 and, although bupropion is subsidised under the Pharmaceutical Benefits Scheme (PBS), nicotine-replacement therapy is not. This contrasts with New Zealand, for example, where a government subsidy for nicotine-replacement therapy, linked to calls to the Quitline, was introduced in 2000.1
One reason for suboptimal investment in smoking-cessation interventions is the paucity of data on the cost savings achievable within specific health budgets. To address this issue, we estimated the potential effect of a smoking-cessation intervention on PBS expenditure using PBS subsidy data and the government’s Intergenerational Report (IGR) model.7
PBS subsidy data for the financial year 2001–02 were obtained from the Department of Health and Ageing for all drugs indicated for the management of cardiovascular disease (CVD) (drugs with Anatomical Therapeutic Chemical codes “C” and “B01”). The distribution of costs by age and sex for the year was assumed to be the same as for May and June 2002, the only two months in the period for which inclusion of the Medicare number on prescriptions was compulsory, and hence age- and sex-specific data were available.
For each drug, the proportion of prescriptions for specific CVDs was estimated from BEACH (Bettering the Evaluation and Care of Health) survey data, in which GPs report the indication for which they are prescribing each drug over the study period.8 A Supplementary Analysis of Nominated Data from BEACH on prescribing of lipid-lowering agents9 and a customised BEACH report on prescribing indications for the specified drugs over the two financial years 2000–02 were used. The former survey covered 1977 GPs and 2661 patient encounters, and found that 41% of patients who were prescribed lipid-lowering therapy had prior ischaemic heart disease. The latter survey covered the same number of GPs and 197 700 patient encounters.
For each CVD, the proportion of costs attributable to tobacco smoking was assumed to be equal to the product of the aetiological fraction for ever smoking tobacco10 and the total cost of subsidies for that disease. Although smoking has not been found to be a risk factor for hypertension, the National Heart Foundation of Australia guidelines recommend considering smoking in management decisions for patients with high blood pressure.11 We assumed that patients who were smokers would be started on antihypertensive medication at a lower blood pressure than non-smokers, based on absolute risk. The proportion of antihypertensive drug costs attributable to smoking was estimated by the proportion of individuals in the Ausdiab study12 who were receiving treatment for hypertension and had either controlled or mild hypertension and were smokers. This proportion was 7%.
PBS subsidies were then estimated for CVD and smoking-related CVD, by age and sex, by therapeutic class and by individual CVD.
Costs for CVD and smoking-related CVD were predicted for each financial year until 2041–42, assuming first that smoking prevalence was unchanged. The government’s forward estimates of total PBS costs were obtained from the IGR, and we assumed that the proportions of PBS costs attributable to CVD and smoking-related CVD remained constant.
Projected smoking-related CVD costs were then recalculated, assuming that an intervention to reduce smoking was started in 2004, ran for 3 years and produced a 5% absolute reduction in smoking prevalence that was sustained until 2041–42. People who stopped smoking as a consequence of the intervention were assumed to have the same risks of CVD as ex-smokers 5 years after the intervention started. CVD risks were assumed to be those of never smokers after 10 years.13 Revised aetiological fractions were calculated with 5% increases in the prevalence of ex-smokers and non-smokers 5 and 10 years, respectively, from the start of the intervention. Projected CVD drug costs were partitioned into smoking-related and other, and the smoking-related costs were recalculated using the revised aetiological fractions. Annual and cumulative differences in smoking-related CVD PBS subsidies under the two assumptions about smoking prevalence were calculated for the 40-year period. Costs were expressed in real (2001–02) dollars, and were also discounted to present value using 5% and 3% annual discount rates.
The government spent $1.29 billion on drugs for CVD in 2001–02, 30.3% of total PBS subsidies. Subsidies for smoking-related CVD were $126 million, 2.96% of total PBS subsidies and 9.77% of CVD drug costs. Males under 65 years of age had the highest costs for smoking-related CVD. Costs for men and women aged 65 years and older were about the same (Box 1).
Ischaemic heart disease accounted for just over 60% of the costs of smoking-related CVD, and hypertension accounted for about a quarter (Box 2). The three most expensive drugs were all statins, comprising 48% of subsidies (Box 3). Note that these figures relate only to statin-prescribing subsidies for patients with prior ischaemic heart disease attributable to smoking.
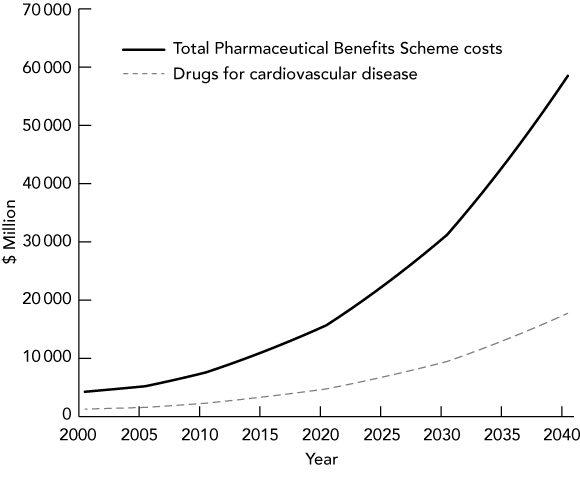
The projected growth in total PBS subsidies and subsidies for drugs to treat CVD over the 40-year period of the IGR are shown in Box 4. The annual cost of the PBS is predicted to increase almost 14-fold, from $4.26 billion to $58.5 billion. The cost of drugs for CVD is predicted to increase from $1.29 billion to $17.7 billion per year by 2041–42.
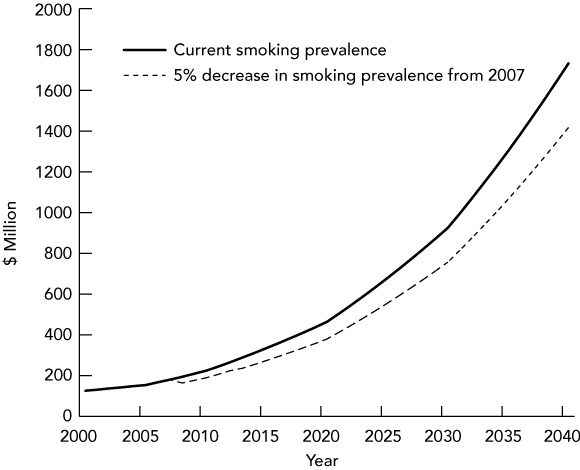
Box 5 shows cost projections for PBS subsidies for drugs to treat smoking-related CVD. Assuming smoking rates do not change, PBS subsidies for smoking-related CVD are predicted to increase to $1.73 billion per year. With a 5% reduction in smoking prevalence, cumulative PBS subsidies would decrease by 17%, with a $4.5 billion reduction in costs for smoking-related CVD over the period, from $25.9 billion to $21.4 billion. When future costs are discounted at 5% per year, a $1.14 billion saving in cumulative PBS subsidies over the period is predicted, a 15.4% reduction, and annual costs in 2041–42 would be $40 million lower. With a 3% per year discount rate, the cumulative saving would be $1.9 billion.
This study had two key findings: our estimate of $126 million for 2001–02 PBS subsidies for smoking-related CVD, and our prediction of a substantial 17% reduction in cumulative PBS subsidies over the 40-year period of the IGR as a consequence of an absolute reduction in smoking prevalence of 5%.
The obvious question is how can this 5% reduction in smoking prevalence be achieved, and at what cost? After tax increases and bans on tobacco advertising, the most cost-effective smoking-cessation intervention is anti-smoking advertising using mass media, particularly television.1,2 Smoking prevalence in Australia had reached a plateau in the 4 years before the 1997 National Tobacco Campaign (NTC). However, it dropped by 1.7% during the initial 7-month phase of the campaign, which launched the now well-known “tumour” and “artery” advertisements. The NTC cost the federal government $7.1 million and the states contributed around $1.85 million. International experience shows that intensive, sustained anti-smoking advertising can produce reductions in smoking prevalence of 5% or more.14
Based on the cost and outcomes of the NTC, we estimate that an enhanced tobacco control program, encompassing a mass media campaign and improved population-level cessation services, would cost $10 million in the first year, $15 million in the second, and $20 million in the third year, and would produce the target 5% reduction in smoking prevalence. We assumed that more intensive (and expensive) advertising over the campaign period would be necessary to achieve further reductions as smoking prevalence declines. If $5 million per year was required to sustain this reduction in smoking prevalence, such a campaign would have an NPV of $1.039 billion, and an internal rate of return (IRR) of 33%, at a 5% per annum discount rate. The payback period would be less than 8 years. At a 3% per annum discount rate, the NPV would be $1.77 billion. Such a campaign would be an excellent investment.
Clearly, our estimates of the costs of this intervention are somewhat arbitrary, but any campaign with a discounted cost of less than $1.14 billion over the 40 years would be cost-saving. Furthermore, our calculations consider only the pharmaceutical costs to treat CVD. The IRR would be far greater and the payback period shorter if other cost offsets were included, for example reduced hospital and medical costs for CVD15 and healthcare costs for myriad other diseases caused or worsened by smoking. The effectiveness of the campaign would also affect its efficiency. We assumed that a 5% reduction in smoking prevalence would occur, based on Australian and international experience. This might vary, but our estimate seems plausible.
Our cost estimation and projection method has limitations. Our disease-costing method is consistent with that of the Australian Institute of Health and Welfare,10,16 but we made the additional assumption that some costs of treating hypertension are attributable to smoking, given the National Heart Foundation of Australia’s guidelines for management of hypertension.11 Further support for this assumption comes from the recently published HOPE study of secondary prevention, which treated people on the basis of absolute risk and reported improvement in cardiovascular outcomes.17
Our cost and cost-saving projections are predicated on the government’s own forward IGR report estimates of PBS costs, which were based on demographic, GDP and spending-growth assumptions.7 The PBS projections were underpinned by a 5.64% per year non-demographic growth rate, which corresponds to the observed real compound growth rate since 1983–84, corrected for the changing age composition of the population. Many assumptions in the IGR could be, and have been, questioned, and it should be noted that its projections related to total PBS subsidies rather than CVD drug costs. Nevertheless, the IGR projections have been used extensively by government as a basis for policy change, such as the proposal to increase patient copayments by almost 30%,18 and the prediction that PBS costs would increase from 0.6% to 3.4% of GDP has raised the question of the PBS’s sustainability. Although there are clearly uncertainties inherent in cost projections spanning 40 years, our model still predicts substantial savings after only 20 years — a 13.5% reduction in cumulative smoking-related CVD drug costs associated with a 5% reduction in smoking prevalence.
Our study demonstrates that anti-smoking interventions have the potential to help control cost pressures on the PBS. Although the projected savings might not be fully realised (as a consequence of changing CVD epidemiology or health system changes, for example), and savings would accrue to the PBS rather than the tobacco control budget, this should not deter implementation of anti-smoking interventions. Smoking-reduction strategies improve health outcomes as well as containing treatment costs borne by government, and therefore offer benefits not achieved through increased patient copayments.
1 PBS subsidies for smoking-related cardiovascular disease
|
Age category |
PBS costs for smoking-related CVD |
Proportion of total |
||||||||||||
Women |
< 65 years |
$31 082 917 |
24.6% |
||||||||||||
Women |
65 years |
$25 946 821 |
20.6% |
||||||||||||
Men |
< 65 years |
$43 048 387 |
34.1% |
||||||||||||
Men |
65 years |
$26 062 650 |
20.7% |
||||||||||||
Total |
|
$126 140 775 |
|
||||||||||||
2 PBS subsidies for smoking-related cardiovascular disease (2001–02), by disease
Disease |
Smoking-related cost |
Proportion of total |
|||||||||||||
Ischaemic heart disease |
$78 868 607 |
62.5% |
|||||||||||||
Hypertension |
$32 673 373 |
25.9% |
|||||||||||||
Heart failure |
$5 566 422 |
4.4% |
|||||||||||||
Cardiac dysrhythmias |
$4 574 841 |
3.6% |
|||||||||||||
Stroke |
$2 947 291 |
2.3% |
|||||||||||||
Atherosclerosis |
$1 503 268 |
1.2% |
|||||||||||||
Pulmonary circulation disease |
$6 973 |
0.01% |
|||||||||||||
Total |
$126 140 775 |
|
|||||||||||||
3 Top 10 drugs by value prescribed for smoking-related cardiovascular disease
Drug |
PBS costs for smoking-related cardiovascular disease |
||||||||||||||
Atorvastatin |
$28 951 938 |
||||||||||||||
Simvastatin |
$24 055 611 |
||||||||||||||
Pravastatin |
$8 415 664 |
||||||||||||||
Clopidogrel |
$5 439 886 |
||||||||||||||
Amlodipine besylate |
$3 817 525 |
||||||||||||||
Irbesartan |
$3 631 027 |
||||||||||||||
Carvedilol |
$3 208 457 |
||||||||||||||
Perindopril |
$2 980 284 |
||||||||||||||
Ramipril |
$2 926 349 |
||||||||||||||
Diltiazem hydrochloride |
$2 897 024 |
||||||||||||||
- Susan F Hurley1
- Michelle M Scollo2
- Sandra J Younie3
- Dallas R English4
- Maurice G Swanson5
- 1 Bainbridge Consultants, North Fitzroy, VIC.
- 2 VicHealth Centre for Tobacco Control, Cancer Council of Victoria, Carlton, VIC.
- 3 Western Australian Division, National Heart Foundation of Australia, Subiaco, WA.
This research was supported with funding from the Victorian Health Promotion Foundation, the Cancer Council Victoria, and the National Heart Foundation of Australia’s National Tobacco Control Program. These funding bodies had no role in the design, conduct or interpretation of the study per se. Susan Hurley is a consultant to the Cancer Council Victoria. Michelle Scollo and Dallas English are employed by the Cancer Council Victoria. Sandra Younie was employed by the Cancer Council Victoria during the conduct of this study. Maurice Swanson is employed by the National Heart Foundation. We thank the following people for their contributions: Thomas Sandeman, Peter Marlton, A/Prof Helena Britt, Dr Jonathan Shaw, Dr Mark Nelson, Dr Andrew Boyden.
None identified.
- 1. VicHealth Centre for Tobacco Control. Tobacco control: a blue chip investment in public health. Melbourne: Cancer Council Victoria, 2001.
- 2. Curbing the epidemic: governments and the economics of tobacco control. Washington: World Bank, 1999. Available at: www1.worldbank.org/tobacco/reports.htm (accessed May 2004).
- 3. Carter R, Scollo M. Economic evaluation of the national tobacco campaign. In: Australia’s national tobacco campaign. Evaluation report volume two. Canberra: Commonwealth Department of Health and Aged Care, 2000; 201-238. Available at: www.health.gov.au/pubhlth/publicat/document/metadata/tobccamp_2.htm (accessed May 2004).
- 4. Applied Economics. Returns on investment in public health: an epidemiological and economic analysis prepared for the Department of Health and Ageing. Canberra: Commonwealth Department of Health and Ageing, 2003. Available at: www.health.gov.au/pubhlth/publicat/document/metadata/roi_eea.htm (accessed May 2004).
- 5. Australian Institute of Health and Welfare. 2001 National Drug Strategy household survey: detailed findings. Drug Statistics Series No. 11. Canberra: AIHW, 2002. (AIHW Catalogue No. PHE-41.)
- 6. Letcher T, Mullins R. Doctors’ advice to their patients about smoking: 1998 update. Melbourne: The Anti-Cancer Council of Victoria, 2000.
- 7. Costello P. 2002–03 Budget Paper No. 5. Intergenerational report 2002–03. Canberra: Commonwealth of Australia, 2002. Available at: www.budget.gov.au/2002-03/bp5/html/index.html (accessed May 2004).
- 8. Britt H, Miller GC, Knox S, et al. General practice activity in Australia 2000–01. General Practice Series No. 8. Canberra: Australian Institute of Health and Welfare, 2001. (AIHW Cat. No. GEP-8.)
- 9. AIHW GP Statistics and Classification Unit. SAND abstract No. 30 from the BEACH program: lipid lowering medications and coronary heart disease. Sydney: GPSCU University of Sydney, 2002. Available at: http://www.fmrc.org.au/Beach/Abstracts/30-Lipid%20lowering%20meds.pdf (accessed May 2004).
- 10. Ridolfo B, Stevenson C. Quantification of drug-caused mortality and morbidity in Australia, 1998. Drug Statistics Series No. 7. Canberra: AIHW, 2001. (AIHW Cat. No PHE-29.)
- 11. National Heart Foundation. Guide to management of hypertension for doctors. Canberra: National Blood Pressure Advisory Committee, 1999.
- 12. Dunstan DW, Zimmet PZ, Welborn TA, et al. The Australian diabetes, obesity and lifestyle study (Ausdiab) — methods and response rates. Diabetes Res Clin Pract 2002; 57: 119-129.
- 13. Doll R, Peto R, Wheatley K, et al. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ 1994; 309: 901-911.
- 14. Biener L, Harris JE, Hamilton W. Impact of the Massachusetts tobacco control programme: population based trend analysis. BMJ 2000; 321: 351-354.
- 15. Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation 1997; 96: 1089-1096.
- 16. Mathers C, Stevenson C, Carter R, Penm R. Disease costing methodology used in the Disease Costs and Impact Study 1993–94. Health and Welfare Expenditure Series No. 3. Canberra: AIHW, 1998. (AIHW Cat. No. HWE-7.)
- 17. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotension-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342: 145-153.
- 18. National Health Amendment (Pharmaceutical Benefits — Budget Measures) Bill 2002–3 (Cwlth), Explanatory Memorandum No. 2.





Abstract
Objective: To estimate Pharmaceutical Benefits Scheme (PBS) subsidies for drugs to treat smoking-related cardiovascular disease (CVD) in 2001–02, and over the period of the government’s Intergenerational Report (IGR), assuming current smoking prevalence rates and a 5% absolute reduction.
Design and setting: An Australian epidemiological study, using prescribing data, aetiological fraction methodology, and IGR trends.
Main outcome measures: Estimated smoking-related PBS subsidy costs in 2001–02 and predicted cumulative subsidies until 2041–42, under current and reduced smoking prevalence assumptions.
Results: The PBS costs of smoking-related CVD in 2001–02 were $126 million, 9.77% of the cost of drugs for CVD and 2.96% of total PBS subsidies. The cumulative difference in these costs over the 40-year period with a 5% drop in smoking prevalence was predicted to be $4.5 billion, a 17% reduction. The saving would be $1.14 billion discounting future costs at 5% per year.
Conclusions: Further investment in tobacco control interventions could curb the increasing cost of the PBS and contribute to government efforts to ensure the viability of Australia’s healthcare-financing programs. The net present value of a campaign to reduce smoking prevalence was estimated at $1 billion, with an internal rate of return of 33%.