A 56-year-old woman presented to her general practitioner a fortnight after a trip to Bali. She had a 2-week history of profuse loose brown stools, lethargy, weakness, nausea and some dyspeptic symptoms. The diarrhoea had initially settled with loperamide, but then recurred.
A faecal specimen was collected for microscopy, culture and testing for enteric viruses, all of which gave negative results. Routine blood tests showed mild iron deficiency with no anaemia. Serum levels of vitamin B12 and folate were in the reference ranges. Serological testing for Toxoplasma and Cytomegalovirus showed no evidence of recent infection. An initial course of metronidazole for presumed Giardia infection was unhelpful, and she was referred for further gastroenterological evaluation.
At the time of review by the gastroenterologist, she had had persistent diarrhoea for nearly 4 weeks. Thyroid, respiratory, abdominal and cardiovascular examination gave unremarkable results. A faecal enzyme-linked immunoassay analysis for Giardia antigens gave negative results. Gastroscopy showed mild diffuse gastritis, and a rapid urease test for Helicobacter pylori was negative. The duodenum appeared normal, and small-bowel biopsy specimens were sent for disaccharidase testing and histopathological examination. No abnormalities were seen on colonoscopy. Faecal fluid was collected and sent in formalin for saline–acid fixation faecal testing.
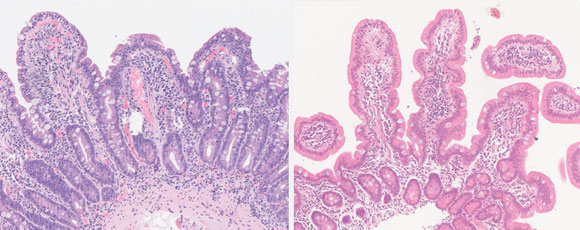
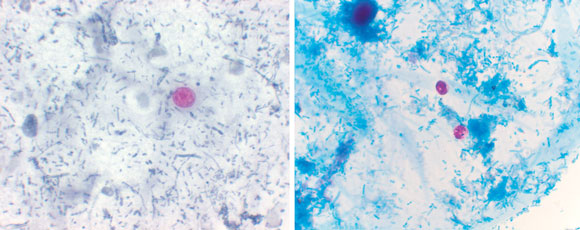
Examination of small-bowel biopsy specimens showed moderate villous blunting with increased intraepithelial and lamina propria lymphocytes and no evidence of dysplasia or malignancy, no granulomas or parasites. The report concluded that this appearance was very suggestive of coeliac disease. (Figure 1). However, examination of the faecal specimen in saline–acid fixative revealed oocytes of Cyclospora cayetanensis (Figure 2).
A diagnosis was made of small intestinal villous atrophy secondary to C. cayetanensis infection. The patient was treated with trimethoprim–sulfamethoxazole (800 mg/160 mg twice daily for 5 days). Her symptoms resolved within days. Serological tests and a repeat small-bowel biopsy were undertaken to exclude latent coeliac disease. Serological testing was negative for antigliadin and antiendomysial antibodies, and there was no evidence of residual villous atrophy in the biopsy specimen (Figure 1). The patient remained well on follow-up after completing the course of antibiotics.
This case of protracted diarrhoea in an Australian traveller initially appeared consistent with coeliac disease. Further analysis revealed infection with Cyclospora cayetanensis, which was successfully treated with antibiotics. The increasing number of documented outbreaks of Cyclospora infection,1 and the need for special preparation of stool samples for their detection,2 highlight the importance of a high degree of suspicion in cases of traveller’s diarrhoea.
C. cayetanensis is a protozoan which was first recognised as a human intestinal parasite in the early 1990s.3 In 1994, Butcher et al described the first case in an Australian traveller of explosive diarrhoea caused by a large acid-fast spherical organism that had only recently been classified as a member of the genus Cyclospora.4 Since then, reports of outbreaks of Cyclospora infection have increased,1 presumably due to greater awareness of this parasite. Outbreaks have been reported from many parts of the world, including North, Central and South America, Europe, South East Asia, India, South Africa, and the Caribbean Islands.5,6
The mode of transmission is thought to be faecal–oral, or via ingestion of contaminated water. The mechanism by which the protozoan causes villous atrophy is not well understood, but organisms have been found at the site of inflammatory changes. Like Cryptosporidium spp. (which are morphologically similar [Figure 2]), Cyclospora spp. cause nausea, profuse diarrhoea and weight loss, as well as profound fatigue. Abdominal pain and bloating can manifest as “indigestion” or “heartburn”. If untreated, symptoms last for 6 weeks to 3 months (longer in the immunocompromised), and can be mistaken for irritable-bowel syndrome. The treatment of choice is trimethoprim–sulfamethoxazole, with ongoing prophylaxis for patients with AIDS.7
Clinicians need to be aware that the histopathological appearance of the small intestine in C. cayetanensis infection is similar to that in coeliac disease. Other causes of small-intestine villous atrophy include viral enteritis, giardiasis, and cows’ milk allergy. Hence, diagnosis of coeliac disease should not be based purely on the finding of villous atrophy. Investigations that we used to test the diagnosis in this patient included a highly specific immunoassay for giardia antigens, as well as testing for antiendomysial and antigliadin antibodies to disprove coeliac disease definitively. However, the clinical history (particularly onset and duration of illness) needs to be considered before requesting these tests. In the setting of recent travel or a community outbreak, a high index of suspicion for infectious causes of villous blunting is warranted.
Despite the increasing knowledge about the genus Cyclospora, many infections are missed, as the parasite can be difficult to detect in human faecal samples. Clinicians need to be aware that acid-fast staining is required, and that this requires preservation of the faecal specimen in formalin.3 It is also important to realise that Cyclospora oocysts are usually shed in low numbers, even when the patient is very ill. In addition, faecal specimens sent to pathology laboratories for examination for ova and parasites are commonly not examined for Cyclospora spp. Hence, Cyclospora testing must be specifically requested.2
Finally, laboratory investigators need to be aware that Cyclospora cysts are morphologically similar to cysts of Cryptosporidium parvum. The most obvious difference is size, as Cyclospora cysts are larger (8–10 μm) than Cryptosporidium cysts (4–6 μm) (Figure 2). It is important to differentiate the two, as Cyclospora infection can be treated with antibiotic therapy, whereas management of Cryptosporidium infection is largely supportive.5 Dialogue between the clinician and pathologist can help to avoid confounding diagnoses. Newer techniques, such as polymerase chain reaction (PCR) testing for parasite DNA, are currently being developed, but are expensive and may not be available in a routine laboratory.
- 1. Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis 2000; 31: 1040-1057.
- 2. Wurtz R. Cyclospora: A newly identified intestinal pathogen of humans. Clin Infect Dis 1994; 18: 620-623.
- 3. Ortega YR, Sterling CR, Gilman RH, et al. Cyclospora species — a new protozoan pathogen of humans. N Engl J Med 1993; 328: 1308-1312.
- 4. Butcher AR, Lumb R, Coulter E, Nielsen DJ. Coccidian/Cyanobacterium-Like Body associated diarrhoea in an Australian traveler returning from overseas. Pathology 1994; 26: 59-61.
- 5. Collins PA, Wright MS. Emerging intestinal protozoa: a diagnostic dilemma. Clin Lab Sci 1997; 10: 273- 278.
- 6. Ortega YR, Sterling CR, Gilman RH. Cyclospora cayetanensis. Adv Parasitol 1998; 40: 399-418.
- 7. Katz DE, Taylor DN. Parasitic infections of the gastrointestinal tract. Gastroenterol Clin North Am 2001; 30: 797-815.





None identified.