Interest in polycystic ovary syndrome (PCOS) has increased recently with the realisation that this syndrome involves far more than the reproductive system. Initially called the Stein–Leventhal syndrome after its researchers in the 1930s, PCOS is now recognised to be a metabolic syndrome which may include hyperinsulinaemia, hyper-lipidaemia, diabetes mellitus, and possibly cardiac disease, as well as the more conventionally recognised increase in androgen levels, cosmetic problems, anovulation, infertility, endometrial cancer and obesity.1,2
The diagnostic criteria for PCOS are controversial (Box 1), but diagnosis is generally based on peripubertal onset of menstrual problems with clinical or biochemical hyperandrogenism. The presence of polycystic ovaries on ultrasound examination is particularly controversial as a criterion. Polycystic ovaries are characterised by peripheral cysts (10 or more) less than 10 mm in size in an enlarged ovary with significant increase in the central stroma4 (Box 2). However, the ultrasound characteristics are subjective. Polycystic ovaries are also found in women with no evidence of menstrual dysfunction or hyperandrogenism.5,6 As polycystic ovaries arise through incomplete follicular development or failure of ovulation, they may also occur in early to mid-adolescence, and in women with bulimia, recovery from anorexia nervosa, conditions of increased adrenal androgen production and hyperprolactinaemia. Most publications on PCOS do not include the presence of polycystic ovaries as a diagnostic criterion.
Several studies have suggested a prevalence of PCOS of 5%–10% in women of reproductive age, using the diagnostic criteria of the US National Institutes of Health. 7,8 Polycystic ovaries alone were found in 20%–25% of women in surveys in the United Kingdom and New Zealand. 5,6 While women with polycystic ovaries and no evidence of menstrual dysfunction or hyperandrogenism appear normal, they do have an overexaggerated response to stimulation with gonadotrophins such as follicle-stimulating hormone (FSH) in cycles of assisted reproduction. PCOS is generally underdiagnosed, and clinicians should remember that menstrual abnormalities, such as cycles shorter than 21 days or longer than 35 days, are often associated with the condition. Many young women with these abnormalities are prescribed the oral contraceptive pill, which masks the condition until they try to achieve pregnancy.
The pathogenesis of PCOS is poorly understood, but the primary defect may be insulin resistance leading to hyperinsulinaemia. In the ovary, the cardinal feature is functional hyperandrogenism (Box 3). Circulating concentrations of insulin and luteinising hormone (LH) are generally raised. The theca cells, which envelop the follicle and produce androgens for conversion in the ovary to oestrogen, are over-responsive to this stimulation. They increase in size and overproduce androgens. The rise in LH levels is thought to be caused by the relatively high and unchanging concentrations of oestrogens that may alter the control of this hormone by the hypothalamic–pituitary axis.
This combination of raised levels of androgens, oestrogen, insulin and LH explains the classic PCOS presentation of hirsutism, anovulation or dysfunctional bleeding, and dysfunction of glucose metabolism. Paradoxically, although the insulin regulatory molecules on the theca cells are responsive to insulin, those in the muscle and liver are resistant.
PCOS is a lifelong condition which may have effects at all ages, not just in the reproductive years (Box 4).
Fetal life. The condition may have its origins in fetal life, with either intrauterine growth retardation or post-term birth. Researchers have claimed that these children are more prone to hyperinsulinism, premature pubarche and signs of PCOS early in reproductive life. 9,10
Teenagers will often have oligo- or amenorrhoea, hirsutism, acne and weight disorders. It is controversial whether patients with PCOS suffer from a raised prevalence of bulimia.
Women seeking to become pregnant will have difficulties because of anovulation and later may be concerned about overweight and hirsutism. It is controversial whether miscarriage is increased in PCOS, or whether pregnancy loss is a result of excess body weight.
Menorrhagia is more common in PCOS because of lack of ovulation and unopposed oestrogen action. The absence of regular menstruation induced by progesterone withdrawal may lead to endometrial hyperplasia and uncontrolled bleeding. There is a theoretical risk of endometrial cancer. Indeed, endometrial cancer has been alleged to be at least four times more common in women with PCOS and may appear in women as young as the early 20s. However, recent studies have raised doubts about the validity of this dogma.11
Features of the metabolic syndrome, including obesity, insulin resistance and dyslipidaemia, are common in women with PCOS.
Obesity: The incidence of obesity in women with PCOS varies between countries and ethnic groups. In the United States, about 50% of women with PCOS are overweight or obese, but this prevalence differs little from that in the general community. In other countries, PCOS appears to be associated with obesity, but at a lower rate than in the US. Obesity tends to be central (abdominal) in its distribution, and even lean women with PCOS may have a fat distribution favouring central omental and visceral fat (Box 5).
Insulin resistance: This is independently related to PCOS, with women of normal weight with PCOS showing a degree of hyperinsulinaemia and impaired glucose disposal after meals and during glucose tolerance tests (oral or intravenous).12 It is uncertain whether this insulin resistance results from a specific genetic post-receptor defect, such as a defect in serine phosphorylation,13 or whether it is comparable to the problem seen in type 2 diabetes. Certainly, hyperinsulinism is common but is difficult to interpret clinically, given the fact that it also results from obesity.
Impaired glucose tolerance and type 2 diabetes: These are major complications in overweight women with PCOS. While fasting glucose level is usually normal, insulin release after a glucose load is increased, and glucose disposal is impaired. An excellent epidemiological study in the UK that followed up women with a histological diagnosis of PCOS after wedge resection of the ovaries found clear evidence of an increase in the rate of diabetes.14 This confirmed the results of many other studies from the US and Europe. In obese women with PCOS, progression from normal glucose function to impaired glucose tolerance or diabetes mellitus is more rapid than in women without PCOS.15
Dyslipidaemia: Hypertriglyceridaemia, increased concentrations of low-density lipoprotein (LDL) cholesterol and decreased concentrations of high-density lipoprotein (HDL) cholesterol are common in women with PCOS, particularly if obese. Levels of plasminogen activator inhibitor-1 may also be raised, suggesting a chronic underlying inflammatory-like process.
Cardiovascular disease: The metabolic features of PCOS have led to widespread concern about the risk of cardiovascular disease. A higher than expected prevalence of PCOS has been reported among young women with angiographically proven narrowing of the coronary vessels; women with PCOS were also more likely to have sonographic evidence of premature obstruction of other large vessels. 16,17 However, a UK study of medical records and death certificates of women with a histological diagnosis of PCOS revealed no evidence for an increase in myocardial infarction or other types of heart disease.14 The association is still under investigation.
History and general examination: These are required to elicit evidence of peripubertal menstrual dysfunction and hirsutism. Gynaecological examination is needed only to exclude other causes of bleeding and miscarriage. Mild clitoromegaly is not uncommon, but significant enlargement raises the possibility of virilisation.
Pelvic ultrasound examination: Transvaginal ultrasound is the best imaging mode. Transabdominal ultrasound examination requires more expertise to get a good view, particularly in obese women. Endometrial thickness should always be assessed to exclude significant endometrial pathology.
Hormone assays: There is ongoing debate about the blood tests needed, if any. Diagnosis of PCOS demands the exclusion of late-onset congenital adrenal hyperplasia (measurement of 17-hydroxyprogesterone), thyroid abnormality (thyroid-stimulating hormone), hyperprolactinaemia (prolactin) and Cushing’s syndrome, but these tests can be omitted if other features are not suggestive. Measurement of testosterone (total or adjusted for sex-hormone-binding globulin) is helpful to show hyperandrogenaemia and to rule out an androgen-secreting tumour. Measurement of other androgens, such as dehydroepiandrosterone sulfate and androstenedione, is not particularly useful.
Glucose testing: It is essential to exclude glucose intolerance with glucose tolerance testing. It is doubtful whether insulin measurement is indicated, as interpretation is clouded by obesity. Some investigators have recommended calculating an index of insulin resistance from glucose and insulin levels (eg, the homeostasis model assessment [HOMA] or quantitative insulin sensitivity check index [QUICKI]).18
Because random and fasting glucose levels are usually normal in women with PCOS, the standard Australian recommendations for diagnosing diabetes by measuring these levels are not applicable, and glucose tolerance testing is recommended.19
Lipid status: Assessment of lipid status is justified (total and HDL cholesterol and triglyceride levels).
Other investigations: Laparoscopy of the pelvis, computed tomography and magnetic resonance imaging are never justifiable for suspected PCOS alone. Endometrial biopsy and hysteroscopy may be used to investigate unexplained vaginal bleeding.
Management comprises treatment of the presenting symptoms, as well as any other abnormalities discovered on investigation. The modality depends on the desire for fertility.
Hirsutism (Box 6) should be assessed qualitatively or semiquantified using the Ferriman–Gallwey score.20 Treatment may include:
the oral contraceptive pill (eg, ethinyloestradiol 35 μg plus cyproterone acetate 2 mg daily for 21 of 28 days);
cosmetic measures (eg, laser electrolysis, bleaching, waxing or shaving);
oral oestrogen and cyproterone acetate (oestradiol valerate 2 mg daily and cyproterone acetate 50 mg for 14 days a month);
spironolactone (75–200 mg daily); or
other drugs, such as the antiandrogen flutamide or the antifungal agent ketoconazole. These drugs either reduce androgen production or inhibit androgen-binding to the receptor. They are not in general use for this purpose in Australia.
Response times for drugs can be up to 3 months.
Menstrual dysfunction, including irregular periods, can be managed by administration of progestins (eg, medroxyprogesterone acetate or norethisterone) or the oral contraceptive pill.
Endometrial hyperplasia should be assessed by ultrasound examination, endometrial biopsy or hysteroscopy, and can be treated by hormonal therapy, such as the oral contraceptive pill or progestins (see case report, Box 7).
Lifestyle changes are a first-line intervention in women with PCOS who are overweight.21 Glucose intolerance can be managed by diet and exercise, weight control and oral antidiabetic drugs (eg, metformin).
The cause of infertility in patients with PCOS is generally lack of ovulation because of a failure of the follicles to develop beyond 10 mm. Most cycles are anovulatory, and induction of ovulation is essential.
Lifestyle modification: Several studies have shown that weight loss can lead to resumption of ovulation within weeks. 22,23 Clark and colleagues demonstrated that even a 5% reduction in body mass restores ovulation and fertility24,25 and devised a program of exercise and sensible eating that has become a model across the world for treating PCOS. Rapid changes in body composition and fat mass can be shown during lifestyle change. High-protein diets seem to be as effective as high-carbohydrate diets, provided that fat and total calories are comparable.26 While lifestyle changes are difficult to maintain, women seeking pregnancy are highly motivated, making this a first-line intervention in overweight women with PCOS. 21,27 Longer-term changes in weight are more difficult to maintain.
Clomiphene citrate: This is an oral oestrogen antagonist that raises circulating concentrations of FSH and induces follicular growth in most women with PCOS and anovulation. The initial regimen is 25–50 mg per day for 5 days. Therapy can be monitored by oestrogen levels, follicular ultrasound examination and luteal progesterone level (> 20 nmol/L). Failure of response is associated with high body mass index and high androgen levels. Doses up to 200 mg per day can be used before failure of response is established. In the rare situation in which side effects limit treatment, tamoxifen can be used. Both treatments increase the risk of multiple pregnancy.
Metformin: Use of the insulin-sensitising drug metformin at doses of 500–2500 mg daily is controversial, but appears valuable in increasing menstrual cyclicity and pregnancy rate. 28-31 A recent consensus statement from the Endocrine Society of Australia indicated its use in women trying to become pregnant.28 Recent systematic reviews suggest that the drug has efficacy for ovulation induction, either as a sole agent or in combination with clomiphene citrate.29 It has been widely used for this purpose, and no specific neonatal complications have been described, despite it being classed as “category C” in Australia (drugs which have caused or may be suspected of causing harmful effects on the human fetus). There is inadequate evidence at present to suggest its use in pregnancy to prevent gestational diabetes or recurrent miscarriage.
The new insulin-sensitising agents, the “glitazones” — troglitazone (now discontinued), rosiglitazone and pioglitazone — have been shown to be very effective for ovulation induction,32 but are not approved by the Pharamaceutical Benefits Scheme for PCOS. There is greater concern about the effects on the fetus of these drugs compared with metformin, and they should not be used by women trying to become pregnant.33
Surgery to the ovaries: Wedge resection of the ovaries has been abandoned because of concerns about pelvic adhesions, another cause of subfertility, and loss of valuable ovarian tissue. Ovarian diathermy or laser drilling has been used in recent years with apparently good results; a recent systematic review comparing drilling with clomiphene citrate and gonadotrophins proved equivalence in the studies examined.34 However, like wedge resection, this surgery may produce pelvic adhesions. Destructive surgery to the ovary should be used only after extensive discussion with the patient and not because the ovaries are found to be polycystic incidentally during routine laparoscopy.
Gonadotrophin treatment: Ovulation induction with gonadotrophins such as FSH has proved successful for at least three decades, but demands skill and experience to avoid multiple pregnancies and ovarian hyperstimulation syndrome. Patients start on low-dose recombinant FSH administered subcutaneously. Monitoring of ovarian response involves ultrasound examination, often with oestradiol measurement. Human chorionic gonadotrophin is given when one follicle reaches 16–20 mm in size. Any more than two follicles of an appropriate size gives the risk of multiple pregnancies. Multiple gonadotrophin cycles may be required to achieve pregnancy, but this approach is preferable before more invasive procedures, such as in-vitro fertilisation.
In-vitro fertilisation: Provided there is no problem other than anovulation, this has little place in the management of infertility resulting from PCOS. Ovulation induction by a skilled reproductive endocrinologist is preferable to in-vitro fertilisation because of the risks of hyperstimulation and multiple pregnancy with the latter procedure.
Women with PCOS require ongoing surveillance to detect impaired glucose tolerance, hyperlipidaemia, endometrial hyperplasia and consequent complications. Obese women, in particular, require regular (possibly annual) glucose tolerance testing because of the potential for rapid progression from normal to impaired glucose tolerance and diabetes.15
Some investigators have suggested prophylactic use of metformin in young teenagers and older women to avoid the problems of the metabolic syndrome. This approach is probably premature at present and is not recommended. Advice about improved exercise and diet is more rational, given the abundant data on the role of lifestyle change in preventing and treating problems of glucose metabolism.
Useful Internet resources for patients are shown in Box 8.
1: Criteria for polycystic ovary syndrome and related disorders
Criteria of the US National Institutes of Health 3
Polycystic ovary syndrome
Presence of menstrual abnormalities and anovulation
Presence of clinical and/or biochemical hyperandrogenaemia
Absence of hyperprolactinaemia or thyroid disease
Absence of late-onset congenital adrenal hyperplasia
Absence of Cushing’s syndrome
Polycystic ovaries
Presence of polycystic ovaries on ultrasound examination
Absence of menstrual or cosmetic symptoms
Absence of biochemical hyperandrogenaemia
Idiopathic hirsutism
Presence of excess hair growth
Absence of biochemical hyperandrogenaemia
Proposed criteria (European Society of Human Reproduction and Embryology and American Society for Reproductive Medicine)*
Polycystic ovary syndrome is diagnosed if there are any two of the following:
Presence of polycystic ovaries on ultrasound examination
Clinical or biochemical hyperandrogenism
Menstrual dysfunction with anovulation
* As concluded at a ESHRE/ASRM-sponsored symposium on PCOS; 1 May 2003; Rotterdam, The Netherlands.
2: Polycystic ovaries
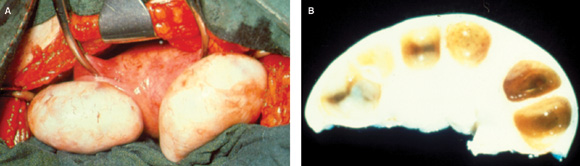
A: Polycystic ovaries, showing increased size and a smooth white surface reflecting thickening of the capsule. B: Section through polycystic ovary, showing multiple cysts with diameter < 10 mm arranged around the periphery of the ovary. The stroma is increased, and the ovary enlarged.
3: Ovarian defect in polycystic ovary syndrome
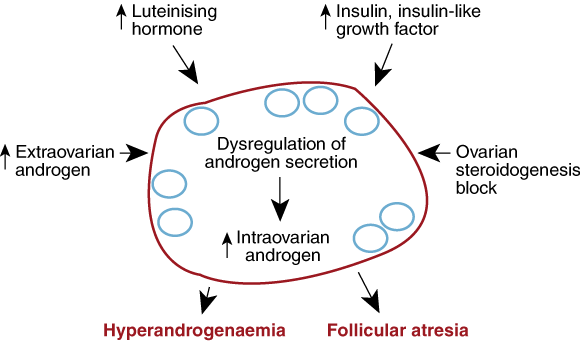
The cardinal feature is functional ovarian hyperandrogenism.
5: Central obesity in polycystic ovary syndrome
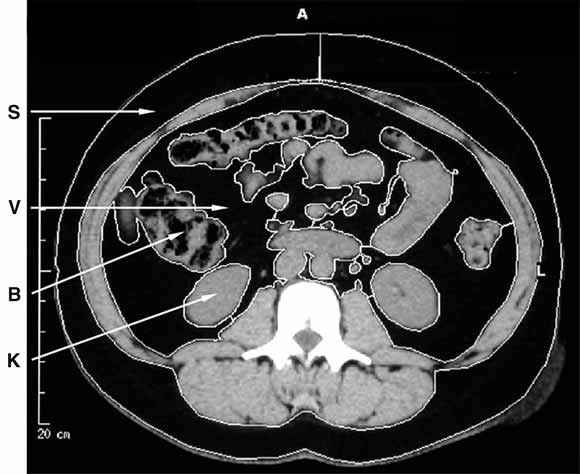
Computed tomography of the abdomen in polycystic ovary syndrome, showing subcutaneous (S) and visceral (V) fat, surrounding bowel (B) and kidneys (K).
6: Skin manifestations of polycystic ovary syndrome
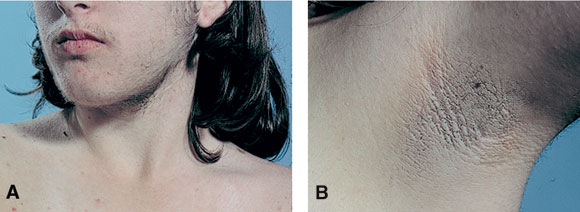
Young woman with PCOS showing facial hirsutism (A) and axillary acanthosis nigricans (B). The latter is associated with severe insulin resistance and hyperinsulinaemia and is an occasional finding in PCOS (photographs courtesy Dr John Casey, St Vincent’s Clinic, Sydney, NSW).
7: Case report — a missed diagnosis
Presentation: A 26-year-old obese single woman presented with severe menstrual bleeding requiring blood transfusion and emergency hormone therapy with progestins. History: She had reached menarche at age 11 years and had irregular periods throughout her teenage years, with menstrual cycles ranging from 6 weeks to 6 months. Menstruation, when it occurred, lasted for up to 4 weeks with heavy clots, and caused fatigue and weakness. She had been admitted to hospital for blood transfusion on three occasions. She complained of hirsutism around the face and lower abdomen and weight gain from the age of 13 years. Two dilatation and curettages a year before had revealed only proliferative endometrium. She had never used regular hormone therapy, such as the oral contraceptive pill or progestins. Investigations: She had significant iron deficiency anaemia (haemoglobin level, 80 g/L; reference range [RR], 115–165 g/L). Tests for clotting abnormalities and thrombophilias gave normal results. Measurement of serum hormone levels showed a raised level of testosterone (3.5 nmol/L; RR, 0.5–1.8 nmol/L), low level of sex hormone binding globulin (15 nmol/L; RR, 20–80 nmol/L), and normal levels of prolactin (300 mIU/L; RR, 150–450 mIU/L) and thyroid-stimulating hormone (2.2 mIU/L; RR, 0.5–4.0 mIU/L). Glucose tolerance testing showed glucose intolerance (2-h glucose level after 75 g glucose load, 10.5 mmol/L). Transvaginal pelvic ultrasound examination showed enlarged polycystic ovaries (ovarian volumes, 12 mL and 15 mL; multiple peripheral cysts < 10 mm on the periphery of the ovaries; and increased ovarian stromal echo) and an enlarged uterus (endometrium, 20 mm). Endometrial biopsy showed proliferative endometrium only. Management: She was treated with medroxyprogesterone acetate (30 mg daily for 2 weeks each month) with minimal response. Six months later, she had an episode of severe bleeding requiring hospital admission. Hysteroscopy and biopsy revealed extensive cystic glandular endometrial hyperplasia with adenomatous changes suggesting an unacceptable risk of malignancy, which necessitated subsequent hysterectomy with ovarian conservation.
|
|
- 1. Norman RJ. Hyperandrogenaemia and the ovary. Mol Cell Endocrinol 2002; 191: 113-119.
- 2. Lobo RA, Carmina E. The importance of diagnosing the polycystic ovary syndrome. Ann Intern Med 2000; 132: 989-993.
- 3. Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, editors. Polycystic ovary syndrome. Boston: Blackwell, 1992: 377-384.
- 4. Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 1986; 293: 355-359.
- 5. Farquhar CM, Birdsall M, Manning P, et al. The prevalence of polycystic ovaries on ultrasound scanning in a population of randomly selected women. Aust N Z J Obstet Gynaecol 1994; 34: 67-72.
- 6. Polson DW, Adams J, Wadsworth J, et al. Polycystic ovaries — a common finding in normal women. Lancet 1988; 1: 870-872.
- 7. Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998; 83: 3078-3082.
- 8. Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999; 84: 4006-4011.
- 9. Ibanez L, Potau N, Ferrer A, et al. Anovulation in eumenorrheic, nonobese adolescent girls born small for gestational age: insulin sensitization induces ovulation, increases lean body mass, and reduces abdominal fat excess, dyslipidemia, and subclinical hyperandrogenism. J Clin Endocrinol Metab 2002; 87: 5702-5705.
- 10. Ibanez L, Potau N, de Zegher F. Ovarian hyporesponsiveness to follicle stimulating hormone in adolescent girls born small for gestational age. J Clin Endocrinol Metab 2000; 85: 2624-2626.
- 11. Hardiman P, Pillay OS, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet 2003; 361: 1810-1812.
- 12. Dunaif A, Segal KR, Futterweit W, et al. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989; 38: 1165-1174.
- 13. Dunaif A. Molecular mechanisms of insulin resistance in the polycystic ovary syndrome. Semin Reprod Endocrinol 1994; 12: 15-20.
- 14. Pierpoint T, McKeigue PM, Isaacs AJ, et al. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol 1998; 51: 581-586.
- 15. Norman RJ, Masters L, Milner CR, et al. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod 2001; 16: 1995-1998.
- 16. Wild S, Pierpoint T, McKeigue P, et al. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf) 2000; 52: 595-600.
- 17. Talbott EO, Guzick DS, Sutton-Tyrrell K, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol 2000; 20: 2414-2421.
- 18. Abassi F, Reaver GM. Evaluation of the quantitative insulin sensitivity index as an estimate of insulin sensitivity in humans. Metabolism 2002; 51: 235-237.
- 19. Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1998; 83: 2694-2698.
- 20. Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961; 21: 1440-1447.
- 21. Norman RJ, Davies MJ, Lord J, et al. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab 2002; 13: 251-257.
- 22. Kiddy DS, Hamilton-Fairley D, Seppala M, et al. Diet-induced changes in sex hormone binding globulin and free testosterone in women with normal or polycystic ovaries: correlation with serum insulin and insulin-like growth factor-I. Clin Endocrinol (Oxf) 1989; 31: 757-763.
- 23. Pasquali R, Antenucci D, Casimirri F, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab 1989; 68: 173-179.
- 24. Clark AM, Ledger W, Galletly C, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod 1995; 10: 2705-2712.
- 25. Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod 1998; 13: 1502-1505.
- 26. Moran LJ, Noakes M, Clifton PM, et al. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003; 88: 812-819.
- 27. Huber-Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab 1999; 84: 1470-1474.
- 28. Norman RJ, Kidson WJ, Cuneo RC, et al. Metformin and intervention in polycystic ovary syndrome. Endocrine Society of Australia, the Australian Diabetes Society and the Australian Paediatric Endocrine Group. Med J Aust 2001; 174: 580-583. <eMJA full text>
- 29. Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev 2003; (3): CD003053.
- 30. Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ 2003; 327: 951.
- 31. Kidson W. Polycystic ovary syndrome: a new direction in treatment. Med J Aust 1998; 169: 537-540. <eMJA full text>
- 32. Azziz R, Ehrmann D, Legro RS, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab 2001; 86: 1626-1632.
- 33. Tarrade A, Schoonjans K, Pavan L, et al. PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J Clin Endocrinol Metab 2001; 86: 5017-5024.
- 34. Farquhar C, Vandekerckhove P, Lilford R. Laparoscopic “drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome (Cochrane Review). The Cochrane Library, Issue 2, 2003. Oxford: Update Software.






Abstract
Polycystic ovary syndrome (PCOS) is a common condition characterised by menstrual abnormalities and clinical or biochemical features of hyperandrogenism.
Features of PCOS may manifest at any age, ranging from childhood (premature puberty), teenage years (hirsutism, menstrual abnormalities), early adulthood and middle life (infertility, glucose intolerance) to later life (diabetes mellitus and cardiovascular disease).
While pelvic ultrasound examination is useful, many women without PCOS have polycystic ovaries; ultrasound evidence is not necessary for the diagnosis.
Testing for glucose intolerance and hyperlipidaemia is wise, especially in obese women, as diabetes mellitus is common in PCOS.
Lifestyle changes as recommended in diabetes are fundamental for treatment; addition of insulin-sensitising agents (eg, metformin) may be valuable in circumstances such as anovulatory infertility.
Infertility can be treated successfully in most women by diet and exercise, clomiphene citrate with or without metformin, ovarian drilling, or ovulation induction with gonadotrophins; in-vitro fertilisation should be avoided unless there are other indications.