People living in areas in Australia with limited access to healthcare services have poorer health than people living in metropolitan areas.1-3 Geographic isolation, poor transport links, shortage of healthcare providers, and more difficult access to healthcare are probably contributing factors, along with a higher proportion of Indigenous people and the generally lower socioeconomic status of residents of remote areas.3
Research on the effect of remoteness on cancer patterns in Australia has largely focused on incidence and mortality rates.3-5 Survival data show that country residents fare worse than metropolitan residents for a wide spectrum of cancers.6,7
Little attention has been paid to the relative contributions of geographic variation in access to cancer screening, diagnostic and treatment services, and geographic variation in cancer survival. Stage at diagnosis differed between cancers diagnosed in urban and rural residents in the United States,8 with later-stage tumours more common in rural residents. This difference is probably due to differences in access to and use and quality of screening and diagnostic services. However, such variation probably runs parallel to variation in access to and quality of treatment services. Both may contribute to geographic variation in survival.8-11 To distinguish between variation in screening and diagnosis and variation in treatment as contributors to variation in cancer survival, measurements of stage of cancer at diagnosis are required. These measurements can then be included in a statistical model of geographical variation in cancer survival.
We describe variation in cancer survival in New South Wales according to a measure of geographic accessibility and remoteness, without and with adjustment for a measure of stage at diagnosis.
The Cancer Council NSW gave ethics approval to analyse data from the NSW Central Cancer Registry, a population-based register of all cancers diagnosed in NSW since 1972.12 Data for 20 different cancer types and all cancers combined were obtained for all patients under the age of 90 diagnosed from 1 January 1992 to 31 December 1996. The cancer types analysed were those for which more than five cases were expected in the remote group.
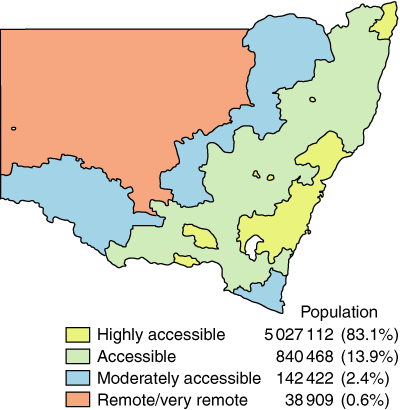
The Accessibility/Remoteness Index of Australia (ARIA) was used to determine remoteness.13 ARIA describes remoteness based on the road distance between a locality and general service centres of various sizes. We obtained ARIA values for each Local Government Area (LGA) in New South Wales and, given the relative rarity of cancer, created four discrete categories: highly accessible, accessible, moderately accessible, and remote (Box 1).
The residential address recorded at the time of diagnosis of cancer was used to allocate each case to an LGA and its corresponding ARIA category.
All cancers were analysed according to their coded “degree of spread of cancer at diagnosis”, except lymphomas, multiple myeloma and leukaemia, for which no measure of stage at diagnosis was available, and primary brain cancer, which was generally localised. The degree of spread was classified as localised, regional (including spread to adjacent organs or regional lymph nodes), distant, or unknown.14 Coding was done either by medical coders in the hospitals who notified the registry, or by medical coders in the registry who used pathology, inpatient and additional reports to make decisions. The distribution of degree of spread at diagnosis (excluding the unknown group) for relevant cancers was compared between the highly accessible category and the remaining categories using an ordinal logistic regression model adjusting for age and sex.
The survival of each cancer patient after diagnosis was determined to 31 December 1999. Weighted probabilistic matching of cancer patients to people in the NSW Death Register and the National Death Index on a range of identifying variables (including full name, sex, date of birth and residential address at diagnosis or death) was used to determine vital status. For patients who were still alive 5 years after diagnosis, or still alive at the end of 1999 with less than 5 years of follow-up, survival times were censored in the analyses.
Five-year relative survival, which measures the excess mortality experienced by patients diagnosed with cancer, was estimated for each cancer type for males and females separately in each ARIA group. Relative survival was calculated as the ratio of the observed probability of dying during the 5 years after diagnosis of the cancer to the expected probability of dying during that period. Expected probability of dying was estimated from the general population death rates for people of the same sex, ARIA group of residence and with the same age distribution as the cancer patients.
The details for estimating expected survival are provided elsewhere.15 Observed survival was determined by the life table method.16 Interval-specific expected survival and cumulative expected survival were estimated using standard methods.17
To model relative survival for each cancer type, the risk of dying at some time (up to 5 years) after diagnosis for people with cancer was modelled as the sum of the expected risk (based on population death rates) and the excess risk due to a diagnosis of cancer. In turn, the logarithm of the excess risk was modelled as a linear function of covariates, including ARIA group, age group, sex and years since diagnosis. The excess risk was modelled for each cancer type using a generalised linear model with binomial errors and a complementary log–log link.16 Additional models were fitted for each cancer type (except brain cancer, lymphomas, multiple myeloma and leukaemia), with stage of cancer at diagnosis included as a covariate. Unknown stage (25.6% of cancer cases on average) was included as an explicit category in this analysis.
Relative excess risk (RER) of death provides a measure for comparing the excess risk of death after a diagnosis of cancer across ARIA groups while adjusting for other variables included in the model. RER was calculated as the exponential of the estimated coefficient corresponding to the ARIA group with the highly accessible group as the reference category (with its RER always equal to one). A relative excess risk > 1 for another category meant that people in that category had a higher risk of excess death than those in the highly accessible group, and vice versa.
The model provided an overall test of statistical significance for differences in the risk of excess death across the ARIA groups after adjusting for the other included variables. Because of the large number of comparisons, a P value of < 0.01 was taken to indicate significance. Ninety-five per cent confidence intervals for the adjusted relative excess risks were calculated from the estimated coefficients and standard errors from the generalised linear model.
There were 108 159 people diagnosed with cancer in the highly accessible group, 20 471 diagnosed in the accessible group, 3143 in the moderately accessible group and 743 in the remote group.
People residing outside the highly accessible areas were more likely to be diagnosed with non-localised cancers of the head and neck, stomach, lung and prostate than people residing in these areas (P < 0.05 for each cancer type).5
When stage of cancer was excluded from the model, there was a 35% excess risk of dying from any cancer in the remote group compared with the highly accessible group (Box 2). The RERs of dying from prostate and cervical cancer in the remote group were nearly three and a half times those in the highly accessible group. People in the moderately accessible group were at a greater risk of dying after diagnosis of lung cancer. There was a notable survival advantage for people diagnosed with cutaneous melanoma and head and neck cancer in the accessible group. For those with head and neck cancer, there was weaker evidence for an increase in the risk of excess death in the remote group, as its 95% confidence interval included 1.
When cancer stage at diagnosis was included in the model, the RER of dying from all cancers combined decreased for the remote category, and the P value for heterogeneity across ARIA groups was larger, but still significant (Box 2). Men in the remote area remained at increased excess risk of death from prostate cancer, and women remained at increased excess risk of death from cervical cancer. The survival advantage for people with melanoma in the accessible group also persisted. The association between residence and poorer lung cancer survival in the moderately accessible ARIA group persisted without much change in the size of the RER or the level of significance. There was a 30% increased excess risk of death from colon cancer for residents in the moderately accessible group. There was also an apparent positive association between remoteness of residence and increased excess risk of death from rectal cancer, but the P value did not reach our criterion of 0.01.
Overall, we found that people living in remote NSW diagnosed with cancer are about 35% more likely to die as a result of their cancer over the ensuing 5 years than are people living in areas with the greatest access to services. This apparent outcome disadvantage is unlikely to be due to chance.
Several issues should be kept in mind when interpreting our results. The unit of aggregation we used, the LGA, is likely to lead to heterogeneity within our remoteness groupings. Furthermore, misclassification of area of residence is possible if some patients living in remote areas moved to more accessible areas for diagnostic tests or treatment and this was recorded as their address at diagnosis. This could explain why, for some cancers, survival appeared better in accessible areas than in highly accessible areas. The operation of these two factors would probably attenuate any positive associations between remoteness of residence and increased relative excess risk of death.
Our measure of the degree of spread of cancer at diagnosis (local, regional, distant metastasis, and stage unknown) is subject to coding and interpretive uncertainties. Degree of spread at diagnosis was unknown for between 26% and 51% of lung, prostate, head and neck and stomach cancers in residents of non-highly-accessible areas. This could reflect poorer reporting from hospitals in these areas, but may also indicate poorer access to specialist oncologists and diagnostic testing, as has been noted elsewhere.8
Insufficient numbers of cases prevented a stage-specific survival analysis of individual cancer sites. However, for all cancers combined, 5-year relative survival was notably worse in the remote group for people with unstaged cancers (data not shown). Interestingly, the trend for poorer survival with increasing remoteness was greatest for cancers diagnosed with regional spread. It is probably in this group that quality of treatment would have the greatest effect. Remoteness may affect treatment choices made by both patients and clinicians,18,19 and this might also affect survival.
The influence of accessibility and quality of services on cancer survival has been examined in several studies. While some show a survival disadvantage with increasing distance from a cancer treatment centre,20 others show that the type of oncology centre and rural health board21 and variation in both treatment factors and the stage of diagnosis9,19 are important explanatory factors. Some suggest that geographic variations in the quality of cancer treatment are more important determinants of survival than factors such as screening and early diagnosis.11,22
Despite significant variation by ARIA group in stage at diagnosis of head and neck, lung, cervical and prostate cancer, control of stage of these cancers reduced the RER to any appreciable extent only for cancers of the cervix and prostate in the most remote areas. This suggests that variation in cancer treatment may be the main determinant of geographic variation in survival for most of these cancers.
The substantial reductions in RERs of prostate and cervical cancer for remote areas when spread of disease at diagnosis was accounted for suggest that screening, diagnosis and treatment deficiencies may all contribute to the excess risks of death for these cancers in remote areas. Issues to consider when interpreting these results are outlined in Box 3.
After adjusting for stage at diagnosis, significant variation by ARIA group was detected for colon cancer. This may be a result of confounding between ARIA group and frequency of screening in a community-based bowel-cancer-screening program, which has been promoted more extensively in non-metropolitan than metropolitan parts of NSW.25
Some factors that may be correlated with geographic location are also correlated with survival, such as socioeconomic status, race and level of education.26,27 In NSW, Indigenous people make up 17.3% of the population of remote areas, as defined in this study, compared with 1.7% of the whole state population. Although accurate data on cancer in Indigenous people are not available in NSW, population-based incidence data elsewhere show higher incidence rates of and poorer survival from some cancers, particularly cancer of the cervix, in Indigenous people in remote areas.28,29 This is in agreement with the differences we have observed.
Poorer access might also explain effects of socioeconomic status (SES) and level of education on survival in similar circumstances, although these factors may have other effects on outcome than those mediated through location. We accounted for SES by using ARIA-specific lifetables to calculate the relative survival (LGAs with lower SES have higher mortality from all causes, thus lower expected survival).
With the possible exception of prostate cancer, our findings of high RERs in less accessible areas for several cancers probably reflect variations in the nature of care received after diagnosis. In NSW, radiation oncology centres are typically located in the highly accessible areas along the middle of the NSW coast. Although many of these centres provide outreach services to larger towns in inland and northern parts of the state, the remote regions are quite distant even from these services.
National conferences of stakeholders involved in non-metropolitan cancer service delivery consistently highlight the need for specialist oncology nurses, improved educational opportunities for staff, and for accommodation and transport support facilities to be addressed.30,31 However, in countries like Australia, with small, widely separated communities outside major metropolitan areas, issues of optimal cost-effective cancer service delivery are complex. It is important to continue exploring ways in which effective consultation, diagnostic support and education32,33 can support the services available in all non-metropolitan areas.
2: Five-year relative excess risk* (95% CI) of death, by Accessibility/Remoteness Index of Australia (ARIA) category for cancers diagnosed in New South Wales from 1992 to 1996
Cancer type |
Without stage of disease as covariate |
With stage of disease as covariate |
||||||||||||
Accessible |
Moderately accessible |
Remote |
P |
Accessible |
Moderately accessible |
Remote |
P |
|||||||
Head and neck |
0.81
|
1.02
|
1.41
|
0.009 |
0.85
|
0.97
|
1.43
|
0.05 |
||||||
Oesophagus |
0.92
|
0.94
|
0.65
|
0.55 |
0.90
|
0.87
|
0.67
|
0.4 |
||||||
Stomach |
1.10
|
1.11
|
0.81
|
0.4 |
1.02
|
1.00
|
0.79
|
0.87 |
||||||
Colon |
1.09
|
1.12
|
0.89
|
0.22 |
1.15
|
1.30
|
1.01
|
0.006 |
||||||
Rectum |
0.98
|
1.25
|
1.78
|
0.1 |
1.07
|
1.22
|
2.32
|
0.02 |
||||||
Liver |
1.22
|
1.24
|
0.79
|
0.41 |
1.22
|
1.35
|
0.78
|
0.39 |
||||||
Pancreas |
1.04
|
0.87
|
1.28
|
0.53 |
1.08
|
0.91 |
1.41
|
0.35 |
||||||
Lung |
1.07
|
1.19
|
1.07
|
0.008 |
1.07
|
1.21
|
1.03
|
0.007 |
||||||
Melanoma of the skin |
0.68
|
1.03
|
0.67
|
0.0004 |
0.71
|
0.92
|
1.04
|
0.002 |
||||||
Breast |
1.10
|
0.91
|
1.21 |
0.46 |
1.11
|
0.89
|
1.47
|
0.24 |
||||||
Cervix |
1.70
|
0.73
|
3.22
|
<0.0001 |
1.73
|
0.83
|
2.25
|
0.0004 |
||||||
Body of uterus |
0.98
|
1.23
|
1.94
|
0.7 |
0.98
|
1.43
|
2.17
|
0.49 |
||||||
Ovary |
1.10
|
0.82
|
1.61
|
0.4 |
1.11
|
0.84
|
1.34
|
0.51 |
||||||
Prostate |
1.18
|
1.44
|
3.38
|
<0.0001 |
1.16
|
1.16
|
2.53
|
0.003 |
||||||
Bladder |
1.06
|
0.95
|
0.75
|
0.85 |
1.10
|
0.94
|
0.60
|
0.5 |
||||||
Kidney |
0.98
|
1.23
|
1.41
|
0.57 |
1.00
|
1.12
|
1.45
|
0.76 |
||||||
Brain |
1.04
|
1.13
|
0.27
|
0.03 |
— | — | — |
— |
||||||
Non-Hodgkin lymphoma |
0.93
|
1.16
|
0.99
|
0.56 |
— |
— |
— |
— |
||||||
Multiple myeloma |
1.08
|
1.11
|
2.67
|
0.31 |
— |
— |
— |
— |
||||||
Leukaemia |
1.01
|
0.98
|
1.40
|
0.82 |
— |
— |
— |
— |
||||||
All cancers |
0.99
|
1.04
|
1.35
|
<0.0001 |
1.02
|
1.02
|
1.25
|
0.003 |
||||||
* Reference is the highly accessible group. All models include age, sex, years since diagnosis and ARIA13 category. — Stage was not available for these cancers. |
||||||||||||||
3: Issues to consider in the interpretation of results for prostate and cervical cancer
Prostate cancer
Uncertainty about efficacy of prostate specific antigen (PSA) screening in lengthening life.23
Relative excess risk (RER) of death may be artefactually high, as frequency of PSA screening is lower in remote areas than accessible areas (unpublished data).
With 51% of cancers of “unknown” stage at diagnosis, adjustment for stage of disease may have been ineffective.
Cervical cancer
Screening is targeted at pre-invasive, not invasive, cancer,24 so changes in RER after adjustment for stage reflect the effectiveness of screening.
Adjustment for stage is likely to have been effective, as it was known for 81.2% of these cancers.
Received 4 July 2003, accepted 19 April 2004
- Katharine E Jong1
- David P Smith2
- Xue Q Yu3
- Dianne L O’Connell4
- David Goldstein5
- Bruce K Armstrong6
- 1 Northern Rivers University Department of Rural Health, University of Sydney, Lismore, NSW.
- 2 Cancer Epidemiology Research Unit, Cancer Council New South Wales, Kings Cross, NSW.
- 3 Department of Medical Oncology, Prince of Wales Hospital, Randwick, NSW.
- 4 School of Public Health, University of Sydney, Sydney, NSW.
Bruce Armstrong’s research is supported by a University of Sydney Medical Foundation Program Grant. NSW Health supported this work by way of a research infrastructure grant. The NSW Central Cancer Registry provided data for this analysis. The registry was managed and operated by the Cancer Council NSW when this work was conducted, but is now based at the NSW Cancer Institute.
None identified.
- 1. Public Health Division. The health of the people of New South Wales. Report of the Chief Health Officer. Sydney: NSW Health, 2000.
- 2. Glover J, Harris K, Tennant S. A social health atlas of Australia. 2nd ed. Volume 1. Adelaide: Public Health Information Development Unit, University of Adelaide, 1999.
- 3. Australian Institute of Health and Welfare. Health in rural and remote Australia. Canberra: AIHW, 1998. (AIHW Cat. No. PHE 6.)
- 4. Lewis N, Nguyen H, Smith DP, et al. Geographic distribution of cancer in New South Wales in 1991 to 1995 by Local Government Area. Sydney: Cancer Council NSW, 1999.
- 5. Jong K, Smith DP, Yu XQ, et al. Remoteness and cancer incidence, mortality and survival in New South Wales 1992 to 1996. Cancer Council NSW, 2002.
- 6. South Australian Cancer Registry. Case survivals by place of residence in Australia. Epidemiology of cancer in South Australia, 1977 to 1998. Adelaide: South Australian Cancer Registry, South Australian Health Commission, 1999: 37-43.
- 7. Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer survival in Australia 1992–1997: geographic categories and socioeconomic status. Canberra: AIHW, 2003. (AIHW cat. no. CAN 17. Cancer Series no. 22.)
- 8. Liff JM, Chow W-H, Greenberg RS. Rural–urban differences in stage of diagnosis. Cancer 1991; 67: 1454-1459.
- 9. Launoy G, Le Coutour X, Gignoux M, et al. Influence of rural environment on diagnosis, treatment and prognosis of colorectal cancer. J Epidemiol Community Health 1992; 46: 365-367.
- 10. Campbell NC, Elliott AM, Sharp L, et al. Rural and urban differences in stage at diagnosis of colorectal and lung cancers. Br J Cancer 2001; 84: 910-914.
- 11. Kim YE, Gatrell AC, Francis BJ. The geography of survival after surgery for colorectal cancer in southern England. Soc Sci Med 2000; 50: 1099-1107.
- 12. Tracey EA, Supramaniam R, Chen W. Cancer in New South Wales: incidence and mortality 2001. Sydney: Cancer Council NSW, 2003.
- 13. Department of Health and Aged Care. Accessibility/ Remoteness Index of Australia (ARIA). Canberra: The Department, March 1999. (Occasional Papers Series No. 6.)
- 14. Esteban D, Whelan S, Laudico A, Parkin DM, editors. Manual for cancer registry personnel. IARC Technical Report No. 10. Lyon: IARC, 1995.
- 15. Voutilainen ET, Dickman PW, Hakulinen T. SURV2: relative survival analysis program. Software manual. Program version 2.02. Helsinki, Finland: Finnish Cancer Registry, 2000.
- 16. Chiang CL. Introduction to stochastic processes in biostatistics. New York: John Wiley, 1968.
- 17. Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations. Methodological note No. 10, End Results Evaluation Section. Bethesda, Md: National Cancer Institute, 1959.
- 18. Kricker A, Haskill J, Armstrong BK. Breast conservation, mastectomy and axillary surgery in New South Wales women in 1992 and 1995. Br J Cancer 2001; 85: 668-673.
- 19. Nattinger AB, Kneusel RT, Hoffman RG, Gilligan MA. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst 2001; 93: 1344-1346.
- 20. Campbell NC, Elliott AM, Sharp L, et al. Rural factors and survival from cancer: analysis of Scottish cancer registrations. Br J Cancer 2000; 82: 1863-1866.
- 21. Howard GCW, Clarke K, Elia MH, et al. A Scottish national mortality study assessing cause of death, quality of and variation in management of patients with testicular non-seminomatous germ-cell tumours. Br J Cancer 1995; 72: 1307-1311.
- 22. Twelves CJ, Thomson CS, Dewar JA, Brewster DH on behalf of the Scottish Cancer Therapy Network. Variation in survival of women with breast cancer: Health Board remains a factor at 10 years. Br J Cancer 2001; 85: 637-640.
- 23. Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet 2003; 361: 1122-1128.
- 24. IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. BMJ 1986; 293: 659-664.
- 25. Rae LC. Community screening for colorectal cancer in north-eastern New South Wales, 1987–1996. Med J Aust 1998; 168: 382-385.
- 26. Bennett CL, Ferreira MR, Davis TC, et al. Relation between literacy, race and stage of presentation among low-income patients with prostate cancer. J Clin Oncol 1998; 16: 3101-3104.
- 27. Lannin DR, Mathews HF, Mitchell J, et al. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA 1998; 279: 1801-1807.
- 28. Condon JR, Armstrong BK, Barnes A, Cunningham J. Cancer in Indigenous Australians: a review. Cancer Causes Control 2003; 14: 109-121.
- 29. South Australian Cancer Registry. Cancer incidence, mortality and case survival in the South Australian Aboriginal population. Epidemiology of cancer in South Australia, 1977 to 1996. Adelaide: South Australian Cancer Registry, South Australian Health Commission, 1997: 11-19.
- 30. Margo J, Goldstein D. Report and recommendations from conference: Cancer in the Bush, Optimising Clinical Services (8–9 March 2001). Canberra: Cancer Council Australia and Australian Department of Health and Aged Care, 2001.
- 31. Kirke K. Living with cancer in Australia. Cancer Forum 2002; 26: 121-122.
- 32. Olver IN, Selva-Nayagam S. Evaluation of a telemedicine link between Adelaide and Darwin to facilitate cancer management. Telemed J 2000; 6: 213-218.
- 33. Dewar AM, Steginga SK, Dunn J, et al. Delivering cancer nursing education to regional, rural and remote area nurses in Queensland. Cancer Forum 2001; 27: 27-29.





Abstract
Objective: To analyse cancer survival in New South Wales by geographic remoteness.
Design, setting and participants: A survival analysis of all patients with cancers diagnosed in NSW between 1 January 1992 and 31 December 1996. Survival was determined to 31 December 1999.
Main outcome measures: The relative excess risk (RER) of death over 5 years was estimated for each geographic remoteness category relative to the highly accessible category for 20 cancer types adjusted for age, sex, years since diagnosis and, subsequently, stage of cancer at diagnosis.
Results: There were statistically significant differences in the RER of death across remoteness categories (P < 0.001) for cancers of the cervix and prostate and for all cancers. The RERs for the most remote categories (compared with the highly accessible category) before and after adjustment for stage were cervix, 3.22 (95% CI, 1.54–6.75) and 2.25 (95% CI, 1.06–4.77); prostate, 3.38 (95% CI, 2.21–5.16) and 2.53 (95% CI, 1.60–4.01); all cancers, 1.35 (95% CI, 1.20–1.51) and 1.25 (95% CI, 1.11–1.41). In addition, there were significant variations in RER of death by remoteness for head and neck, lung and colon cancers and cutaneous melanoma.
Conclusion: Cancer survival varies by remoteness of residence in NSW for all cancers together and some cancers individually. Access to screening or early diagnosis probably contributes to this variation, but persistence after adjustment for stage suggests that treatment variation is also important.