The malaria-receptive zone in northern Australia, defined more than 50 years ago,1 tends to coincide with the distribution of most important malaria vectors in Australia — the sibling species of the Anopheles farauti sensu lato (s.l.) complex.2 Although the receptive zone is extensive, and there are numerous importations of malaria into the zone from overseas each year,3 locally acquired malaria is rare in Australia.
Malaria was eradicated from Australia by 1981, and the World Health Organization declared the country malaria free in 1983.4 Since then, there have been only three documented episodes of malaria acquired in mainland Australia, all caused by Plasmodium vivax, and all in Far North Queensland. Two episodes involved single cases,5,6 but the third, in 1986, was an outbreak that included six locally acquired cases.4 We report here a second outbreak of multiple cases of P. vivax malaria, acquired at a camping ground in Far North Queensland in October 2002.
Laboratories in Queensland are required to notify to local public health authorities patients diagnosed with malaria by microscopic examination. The diagnosis is confirmed by the Malaria Reference Laboratory in the Haematology Laboratory, Royal Brisbane and Women’s Hospital, Brisbane, Queensland.
The Tropical Public Health Unit in north Queensland ascertains the likely source of each case of malaria notified in north Queensland from the travel history. If the diagnostic laboratory reports gametocytes (the stage in the life-cycle of the parasite that is infectious to mosquitoes) in the patient’s peripheral blood, then the patient’s travel history in north Queensland before treatment is also sought.
As soon as this outbreak and its association with the camping ground were recognised in late October 2002, all infected people were questioned about their stay at the camping ground and use of mosquito repellents of any type by themselves and other members of their camping parties. Further cases were sought by contacting, where possible, all parties registered to stay at the camping ground with the Queensland Parks and Wildlife Service (the agency responsible for managing the camping ground) during the first 3 weeks of October 2002. As many of the campers and some notified patients were from overseas, a request for details of any other possible cases diagnosed abroad was posted on ProMED, an electronic communicable diseases bulletin board,7 in early November.
Larval and adult mosquito surveys were conducted at the camping ground in late October 2002. Mosquito larvae were sampled with a 350 mL dipper, and adult mosquitoes were trapped with US Centers for Disease Control light traps baited with 1 kg of dry ice. Five light traps were set overnight in rainforest, at about equidistant intervals along the north–south length of the camping ground (Box 1).
Anopheles mosquitoes were identified using morphological keys,8 and sibling species were identified using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis.9 Adult female Anopheles mosquitoes were screened for malaria sporozoites using a dipstick assay (Vectest malaria sporozoite antigen panel assay, Medical Analysis Systems, Camarillo, Calif, USA).10
The insect growth regulator S-methoprene was used to treat pools where Anopheles larvae were found, and the residual pyrethroid insecticide deltamethrin was applied with a pump sprayer to densely vegetated areas within the camping ground.
To determine the relationships between P. vivax isolates in the different cases, we characterised segments of the genes encoding the apical membrane antigen-1 (AMA1) and merozoite surface protein-1 (MSP1)11 of the parasites.
Parasite DNA was extracted from whole blood samples12 that had been stored at − 70°C. The AMA1 and MSP1 genes were amplified by PCR using the primers PvAF5 and PvAR11 for AMA1, and MF3 and MR1 for MSP1,11 and AmpliTaq Gold DNA polymerase (Perkin Elmer, Branchburg, NJ, USA). Three PCR reactions were performed for each blood sample.
PCR amplicons were purified for direct nucleotide sequencing using a Wizard PCR Preps kit (Promega, Madison, Wis, USA). Sequencing reactions were carried out in a thermal cycler using dye terminator reaction and were analysed using a 377 DNA sequencer and the SeqEd program (PE-ABI, Foster City, Calif, USA). Two to three PCR products were sequenced for each genotype determination.
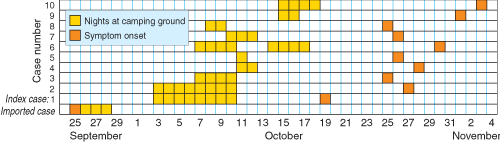
The index (ie, first recognised) case was in a 29-year-old man reported to the Tropical Public Health Unit in Cairns on 29 October 2002 with P. vivax malaria. Although he had visited South-East Asia and India several times, he had not been overseas since 1998, and he had never had malaria before. His two adult household companions, one of whom had never been overseas, had similar febrile illnesses. Within 24 hours, these two were also confirmed with P. vivax malaria, indicating an outbreak of at least three locally acquired cases.
In the 2 weeks before onset in the index patient, the three had been together overnight at two different locations: a residence near Cairns, in an area known to be low-risk for Anopheles mosquitoes; and at the Noah Beach camping ground within the Daintree National Park, about 95 km north of Cairns. There they slept in the open, and reported many mosquitoes, particularly in the evenings.
A month before notification of the index case, the Unit had been notified of a 57-year-old man with imported (ie, acquired outside Australia) P. vivax malaria. He had previously had P. vivax malaria in July 2001, after a trip to Indonesia in May 2001, during which he had not complied with mefloquine chemoprophylaxis. On that occasion, he was treated with chloroquine (as recommended13) and primaquine (15 mg orally daily) for an unspecified period. In January 2002, he travelled to Madagascar, and was compliant with mefloquine chemoprophylaxis during that trip.
He had become unwell in late September 2002 and had presented at the hospital in Cooktown, about 70 km north of Noah Beach. As it was not known then that he had stayed overnight at Noah Beach, the initial public health response focused on Cooktown. However, after recognition of the outbreak, further questioning revealed that he had stayed at Noah Beach for 4 nights while unwell. Moreover, gametocytes were reported in his blood film by the diagnostic laboratory. Assuming duration of P. vivax sporogony is about 12–14 days at the average daily temperature in September in Far North Queensland (26°C),14 any Anopheles mosquitoes infected by this man would have been capable of infecting other people from about 8 October.
Over 2 weeks, 10 cases of P. vivax malaria after overnight stays at Noah Beach were notified to the Unit (Box 2). One case was diagnosed and notified from interstate, and four were in overseas visitors, but no further reports were received from abroad. All patients were adults (age range, 21.5–43.5 years), and seven were men. The patients became ill 8–20 days after leaving the camping ground, with maximum possible incubation periods of 11–22 days; one patient, who stayed 1 night at Noah Beach, had an incubation period of 15 days.
Five of the eight parties camped at the northern end of the camping ground, two camped in the middle near the toilet block, and one spent a night in the middle and another 3 nights at the northern end (Box 1). Five parties reported sleeping in situations that would have exposed those infected, as well as some who remained well, to night-biting mosquitoes: sleeping in the open, in a tent with legs protruding outside, in a tent with damaged netting, or in a vehicle with open windows. The use of mosquito repellents could be ascertained for 26 individuals in seven parties. Eight of the nine infected patients did not use repellents routinely (or at all) compared with 16 of the 17 well individuals who used repellents appropriately (odds ratio, 0.01; exact 95% CI, 0.00–0.19; Fisher’s exact test, P < 0.001).
The Noah Beach camping ground lies in a lowland rainforest and is bounded by two small creeks, which had been reduced by prolonged dry weather to a series of pools cut off from the ocean by dunes.
The north creek pools (salinity, 9 parts per thousand) were edged by mangroves and pandanus, and covered by a thick layer of Rhizophora leaves (Box 3). Large numbers of An. farauti s.l. larvae (5 to > 50 per 350 mL dipper) were collected in these pools. The south creek pools contained fresh water with an extensive cover of floating pumice stone, potentially protecting mosquito larvae from predatory fish. Anopheles larvae were found at densities of 1–5 larvae per dipper (75% identified as Anopheles bancroftii and 25% as An. farauti s.l.). Both sites were treated with S-methoprene pellets on 1 November 2002.
A total of 940 female mosquitoes were collected overnight from five light traps set at the camping ground; 782 of these mosquitoes (83%) were identified as belonging to An. farauti s.l. (Box 1). All 366 An. farauti s.l. mosquitoes tested by PCR-RFLP analysis were An. farauti sensu stricto (s.s.; formerly classified as An. farauti no. 115). Most adult female An. farauti s.l. were trapped near the north creek and the toilet block. None of 706 mosquitoes tested by the dipstick assay were positive for malaria sporozoites.
Sequencing of the AMA1 and MSP1 PCR products from different cases showed a range of genetically distinct forms (haplotypes) of P. vivax, characterised by specific point mutations12 (Box 4). A blood sample taken from the imported case in July 2001, during this patient’s first clinical episode of malaria, yielded two haplotypes that shared the same MSP1 genotype (termed 1-MSP1) but differed in their AMA1 genotypes (1-AMA1 and 2-AMA1). Products of individual PCR reactions contained only single AMA1 genotypes, but different genotypes were present in different PCR products from the same sample. Multiple PCR products showed the same MSP1 allele.
Neither of these haplotypes was detected in the blood sample taken from this man in September 2002, after his trip to Madagascar. Rather, a third haplotype, distinguished by its MSP1 genotype (2-AMA1 : 2-MSP1), was detected. This same haplotype was found in four of the eight outbreak cases that had PCR sequencing. A fourth haplotype was seen in another two outbreak cases (1-AMA1 : 2-MSP1); this had the same MSP1 genotype as the other outbreak cases, but the AMA1 genotype found in the imported case in 2001. In another two outbreak cases, only one of the two genes could be amplified, despite repeated reactions.
Although the outbreak of P. vivax malaria reported here was short and intense, it was not sustained and did not lead to further transmission. There are two likely reasons. Firstly, the camping ground had no permanent residents, and most campers stayed for only a couple of days. Therefore, once the imported case departed, there was no reservoir of infected people at the camping ground to infect later generations of mosquitoes.
Secondly, because the average longevity of an adult female Anopheles mosquito in the tropics is about 10–14 days,16 only a very small percentage of infected mosquitoes survive long enough to infect people. The daily survival of An. farauti s.l. is about 75% near Darwin17 and about 70% in coastal Papua New Guinea.18 Assuming similar survival, only about 0.008%–0.0008% of the mosquitoes infected by the patient with imported malaria on his last evening at the camping ground would have still been alive for the entomological survey 33 days later. Therefore, the failure of the dipstick assay to detect sporozoites in mosquitoes collected at that time was expected.
The mosquitoes collected at the camping ground were predictably identified as An. farauti s.s., as this is the only salt tolerant member of the An. farauti s.l. complex in Australia.19 The distribution of mosquitoes in the camping ground, with high densities in the middle and at the north end was consistent with the infected patients’ camp sites. Indeed, no infected person had camped at the south end, where mosquito densities appeared lowest.
The PCR sequencing strategy revealed a multiple infection in the patient with imported malaria, and highlighted an unusual variety of genotypes in the outbreak cases. The patient with imported malaria received an inadequate dose and possibly duration of primaquine treatment after his initial infection (acquired in Indonesia) to eliminate the P. vivax strains prevalent in South-East Asia.13 However, although his initial multiple infection may not have been adequately cleared, neither haplotype was detected during his second episode of P. vivax malaria.
The haplotype that was detected in this patient in his second malaria episode (2-AMA1 : 2-MSP1) was clearly implicated in the outbreak. Although the other haplotype implicated in the outbreak (1-AMA1 : 2-MSP1) was not detected in the imported case during either malaria episode, its component alleles were detected: 1-AMA1 in blood taken during the first episode, and 2-MSP1 during the second episode.
A possible explanation is that the patient with imported malaria had a multiple infection with two haplotypes in September 2002, one of which was not detected by PCR. Differential amplification of diverse alleles by PCR has been previously described in infections with multiple haplotypes of Plasmodium falciparum.20 This multiple infection may have included the 1-AMA1 : 2-MSP1 haplotype found in the two outbreak cases. Alternatively, it may have included the 1-AMA1 : 1-MSP1 haplotype from this patient’s original malaria episode. The outbreak haplotype could then have resulted from sexual recombination between gametocytes during meiosis in the gut of mosquitoes infected by this patient at Noah Beach. While sexual recombination has been observed in experimental crosses of two distinct laboratory strains,21 it has not previously been documented in the field.
If this hypothesis is correct, then the parasite that caused the outbreak probably originated in Indonesia, as at least three of the four alleles found in the two outbreak haplotypes were present in the initial infection of the imported case. This suggests that the outbreak may have been prevented if the imported case had been treated with the appropriate primaquine dosage to clear latent hepatic stages during the initial malaria episode.
Although there may have been reporting bias, it is clear that most infected patients not only had put themselves at risk of mosquito bites by exposing body parts at night, but also had not used insect repellents appropriately. Conversely, those who used repellents appeared effectively protected from malaria. Repellents, particularly those containing diethyl methylbenzamide (DEET), appear effective in protecting people from mosquito bites for considerable periods.22,23 Campers in north Queensland need to be aware that they have an increased risk of mosquito-borne diseases, which also notably include those caused by alphaviruses, such as Ross River virus.24 They should always adopt mosquito protection measures, such as effective tent screens and routine use of effective repellents.
This outbreak was remarkably similar to the only other recognised mainland outbreak with multiple cases since malaria was eradicated from Australia in 1981. That outbreak, in 1986, also occurred in the Daintree National Park, at a camping area just north of Noah Beach that is no longer used; most transmission also occurred in October.4 Presumably, the risk of local transmission in Far North Queensland is higher in the dry season, when creeks are reduced to stagnant pools and high ambient temperatures increase Anopheles populations.
However, the risk of malaria is very low, and local transmission remains rare in this region, despite frequent importations. We confirm our belief that malaria does not pose an important threat to the health of the public in Far North Queensland.3 Nevertheless, it remains an important issue for travellers to malarious areas, and for clinicians and laboratories throughout Australia.
1: Site associated with the malaria outbreak
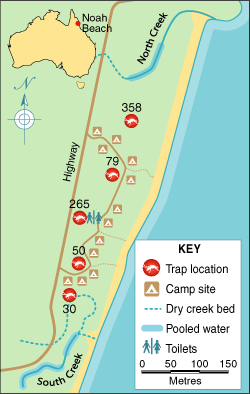
The Noah Beach camping ground in Far North Queensland, showing camp sites, creeks, and numbers of adult female Anopheles farauti sensu lato mosquitoes trapped overnight in light traps in late October 2002.
3: Noah Beach camping ground, Far North Queensland

Camp sites and a remnant pool in the north creek, which contained numerous larvae of Anopheles farauti sensu lato.
4: Haplotypes of Plasmodium vivax from the patient with imported malaria and the outbreak cases
Patient |
Date of blood sample |
P. vivax haplotype* |
|||||||||
Imported case |
Jul 2001 |
1-AMA1 : 1-MSP1, 2-AMA1 : 1-MSP1 |
|||||||||
|
Sep 2002 |
2-AMA1 : 2-MSP1 |
|||||||||
Outbreak cases† |
|
||||||||||
Index (1) |
Oct 2002 |
2-AMA1 : 2-MSP1 |
|||||||||
3 |
Oct 2002 |
2-AMA1 : 2-MSP1 |
|||||||||
4 |
Oct 2002 |
2-AMA1 : 2-MSP1 |
|||||||||
5 |
Oct 2002 |
1-AMA1 : –‡ |
|||||||||
6 |
Nov 2002 |
1-AMA1 : 2-MSP1 |
|||||||||
8 |
Nov 2002 |
1-AMA1 : 2-MSP1 |
|||||||||
9 |
Nov 2002 |
– : 2-MSP1‡ |
|||||||||
10 |
Nov 2002 |
2-AMA1 : 2-MSP1 |
|||||||||
* Haplotype was classified according to the sequences (type 1 or type 2) of the AMA1 and MSP1 genes. † No samples were available for Cases 2 and 7. ‡ Only one of the genes could be amplified for Cases 5 and 9. |
|||||||||||
- Jeffrey N Hanna1
- Scott A Ritchie2
- Dianne L Brookes3
- Brian L Montgomery4
- Damon P Eisen5
- Robert D Cooper6
- 1 Tropical Public Health Unit, Queensland Health, Cairns, QLD.
- 2 Infectious Diseases Unit, Royal Brisbane Hospital, and Malaria and Arbovirus Unit, Queensland Institute of Medical Research, Brisbane, QLD.
- 3 Army Malaria Unit, Enoggera, QLD.
We wish to thank all those involved in the investigations into the outbreak, particularly the rangers of the Queensland Parks and Wildlife Service who assisted us to locate many of the camping parties.
None identified. No external funding was sought for investigations into the outbreak.
- 1. Ford E. The malaria problem in Australia and the Australian Pacific Territories. Med J Aust 1950; 1: 749-760.
- 2. Cooper RD, Frances SP, Waterson DGE, et al. Distribution of anopheline mosquitoes in northern Australia. J Am Mosq Control Assoc 1996; 12: 656-663.
- 3. Hanna JN, Brookes DL, Ritchie SA, et al. Malaria and its implications for public health in Far North Queensland: a prospective study. Aust N Z J Public Health 1998; 22: 196-199.
- 4. Walker J. Malaria in a changing world: an Australian perspective. Int J Parasitol 1998; 28: 947-953.
- 5. Brookes DL, Ritchie SA, van den Hurk AF, et al. Plasmodium vivax malaria acquired in far north Queensland. Med J Aust 1997; 166: 82-83. <MJA full text>
- 6. Jenkin GA, Ritchie SA, Hanna JN, Brown GV. Airport malaria in Cairns. Med J Aust 1997; 166: 307-308.
- 7. Hugh-Jones M. Global awareness of disease outbreaks: the experience of ProMED-mail. Public Health Rep 2001; 116 Suppl 2: 27-31.
- 8. Lee DJ, Hicks MM, Griffiths M, et al. The Culicidae of the Australasian region. Vol 5. Canberra: AGPS, 1987.
- 9. Beebe NW, Saul A. Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction — restriction fragment length polymorphism analysis. Am J Trop Med Hyg 1995; 53: 478-481.
- 10. Ryan JR, Dav K, Emmerich E, et al. Dipsticks for rapid detection of plasmodium in vectoring anopheles mosquitoes. Med Vet Entomol 2001; 15: 225-230.
- 11. Figtree M, Pasay CJ, Slade R, et al. Plasmodium vivax synonymous substitution frequencies, evolution and population structure deduced from diversity in AMA 1 and MSP 1 genes. Mol Biochem Parasitol 2000; 108: 53-66.
- 12. Eisen D, Billman-Jacobe H, Marshall VF, et al. Temporal variation of the merozoite surface protein-2 gene of Plasmodium falciparum. Infect Immun 1998; 66: 239-246.
- 13. Antibiotic Writing Group. Malaria. In: Therapeutic guidelines: antibiotic. Version 11. Melbourne: Therapeutic Guidelines Ltd, 2000: 95-100.
- 14. Sinden RE, Gilles HM. The malaria parasites. In: Warrell DA, Gilles HM, editors. Essential malariology. 4th ed. London: Arnold, 2002: 8-34.
- 15. Schmidt ER, Foley DH, Hartel GF, et al. Descriptions of the Anopheles (Cellia) farauti complex of sibling species (Diptera: Culicidae) in Australia. Bull Entomol Res 2001; 91: 389-411.
- 16. Service MW, Townson H. The Anopheles vector. In: Warrell DA, Gilles HM, editors. Essential malariology. 4th ed. London: Arnold, 2002: 59-84.
- 17. Russell RC. Seasonal abundance, longevity and population age composition of potential malaria vectors in northern and southern Australia. Aust J Zool 1989; 35: 289-306.
- 18. Charlwood JD. Survival rate variation of Anopheles farauti (Diptera: Culicidae) between neighboring villages in coastal Papua New Guinea. J Med Entomol 1986; 23: 361-365.
- 19. Sweeney AW. Larval salinity tolerances of the sibling species of Anopheles farauti. J Am Mosq Control Assoc 1987; 3: 589-592.
- 20. Eisen DP, Saul A, Fryauff DJ, et al. Alterations in Plasmodium falciparum genotypes during sequential infections suggest the presence of strain specific immunity. Am J Trop Med Hyg 2002; 67: 8-16.
- 21. Ranford-Cartwright LC, Balfe P, Carter R, Walliker D. Frequency of cross-fertilization in the human malaria parasite Plasmodium falciparum. Parasitology 1993; 107: 11-18.
- 22. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med 2002; 347: 13-18.
- 23. Frances SP, Cooper RD, Sweeney AW. Laboratory and field evaluation of the repellents deet, CIC-4, and AI3-37220 against Anopheles farauti (Diptera: Culicidae) in Australia. J Med Entomol 1998; 35: 690-693.
- 24. Harley D, Sleigh A, Ritchie S. Ross River virus transmission, infection and disease: a cross-disciplinary review. Clin Microbiol Rev 2001; 14: 909-932.





Abstract
Objective: To describe an outbreak of Plasmodium vivax malaria in Far North Queensland in 2002.
Design: Epidemiological and entomological investigations; molecular analyses of the infecting parasites.
Main outcome measures: Case characteristics, adult and larval mosquito counts at the outbreak location, haplotyping of parasites in blood samples from different cases determined through sequencing of AMA1 and MSP1 genes.
Results: A man with imported P. vivax malaria stayed at a camping ground 95 km north of Cairns in late September 2002. This led to an outbreak of P. vivax malaria in 10 adults who stayed at the camping ground in October. Large numbers of Anopheles farauti sensu lato larvae were present in stagnant pools in a creek at the camping ground, and many adult mosquitoes were collected nearby. Not only had most of the infected patients been exposed to mosquitoes at night, they were also less likely than other campers to have used insect repellents appropriately (odds ratio, 0.01; P < 0.001). Two different haplotypes of P. vivax, only one of which was detected in the imported case, were involved in the outbreak.
Conclusions: Although local transmission of malaria is rare in Far North Queensland, the risk is probably higher in the dry season (September to December). Campers need to be aware of the increased risk of mosquito-borne diseases. Sexual recombination of multiple gametocytes in mosquitoes infected by the imported case may have resulted in the two haplotypes of P. vivax involved in the outbreak.