A 71-year-old white woman presented with increased shortness of breath over a 6-day period, followed by acute onset of severe back and interscapular pain. Her past medical history consisted of hypertension, type 2 diabetes, and a distant history of alveolar-cell carcinoma of the lung, with right lower lobectomy in 1974.
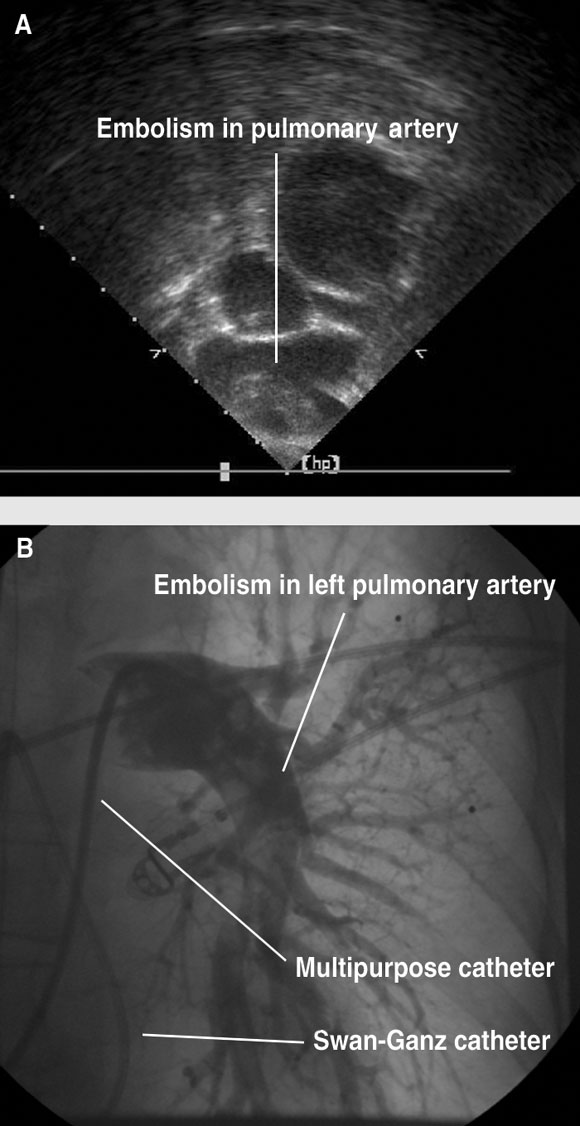
Physical examination revealed an obese lady weighing 95 kg, with a blood pressure of 80/40 mmHg, pulse 110/min, and a respiratory rate of 36/min. The patient was afebrile, diaphoretic and restless. Her venous pressure was elevated. Chest examination showed reduced breath sounds at both lung bases. Heart sounds were dual with no murmurs. Electrocardiography showed sinus tachycardia, 110/min with an S wave in lead I, a Q wave in limb lead III, and T-wave inversion in limb lead III. Arterial blood analysis (inspired oxygen content of 21%) showed pH, 7.156; Pao2, 61 mmHg; Paco2, 52 mmHg; Sao2, 83%. The patient’s chest x-ray showed a widened mediastinum on a mobile supine film, and previous right lower lobectomy. The provisional diagnosis was aortic dissection. The patient was intubated and ventilated in the emergency room, then taken to the intensive care unit, where she was resuscitated with intravenous fluids (both crystalloid and colloid) and an infusion of adrenaline. Transoesophageal echocardiography showed a small left ventricle with hyperdynamic systolic function. The right ventricle was dilated, with poor systolic function. There was grade 2/4 tricuspid regurgitation, with an estimated right ventricular systolic pressure of 60 mmHg. There was grade 3/4 mitral regurgitation secondary to systolic anterior motion of the anterior mitral leaflet, and dynamic left ventricular outflow tract obstruction with a maximum gradient of 90 mmHg. Significant thrombus was seen in the proximal right and left pulmonary arteries (Box 1A). There was no evidence of aortic dissection and no pericardial effusion. Other laboratory results included elevated D dimer level of 2.93 mg/L (normal, < 0.28 mg/L), with normal serum creatine kinase and cardiac troponin I levels.
The patient was given 7000 U of unfractionated heparin and thrombolysis with 40 mg of intravenous tenecteplase administered twice, but she remained profoundly hypotensive (BP, 78/47 mmHg). Surgical embolectomy was considered, but was declined due to the recently administered thrombolysis. A decision was made to take the patient to the cardiac catheterisation laboratory for an attempt at mechanical intervention.
While the laboratory was being prepared, a pulmonary artery flotation catheter was passed from the right internal jugular vein into the main pulmonary artery. This resulted in significant clearance of the “saddle” embolism from the main pulmonary artery, as determined by transoesophageal echocardiography.
The time from administration of thrombolysis to commencement of the interventional procedure was 55 minutes. The activated clotting time, measured when femoral access was obtained, was 352 seconds. The main pulmonary artery was accessed via the right femoral vein with a multipurpose catheter. As a rheolytic thrombectomy catheter and other commercial devices were not available, an Amplatz 0.035" wire was used to remove the multipurpose catheter and insert a 7 Fr long sheath and a pigtail catheter. With the pigtail catheter, the embolism was mechanically cleared from the main pulmonary artery. A pulmonary angiogram then showed extensive embolism in the left main pulmonary artery, extending into several lower lobe segmental arteries (Box 1B). The pigtail catheter was used to macerate this embolism. Once some blood flow had been restored, a snare was then made using an 0.025" wire, but attempts to snare the clot were not successful — the clot was pushed further into the segmental arteries. Nevertheless, fragmentation of the embolism and clearance of the main pulmonary artery and left pulmonary artery was achieved. A multipurpose catheter was then passed to the right pulmonary artery and the stiff wire was used to remove the multipurpose catheter and insert the 7 Fr long sheath and a pigtail catheter. Pulmonary angiography of the right lung showed the right main pulmonary artery to be clear of thrombus, which had lodged in the right segmental branches.
At the completion of the intervention, the systemic blood pressure had stabilised (90/54 mmHg) and the pulmonary pressures were only moderately elevated (44/24 mmHg). Angiography showed that blood flow through the lungs had improved. Anticoagulation was continued with intravenous unfractionated heparin. The activated partial thromboplastin time 4 hours after the intervention was > 250 seconds.
The following day the patient was extubated and discharged to the ward. Her dyspnoea and mobility gradually improved, and she was started on warfarin. Four days after the procedure, transthoracic echocardiography showed the right ventricle to be at the upper limit of normal in size, with normal systolic function. The right ventricular systolic pressure was estimated at 62 mmHg.
Acute major pulmonary embolism is associated with right ventricular dysfunction and shock.1 This condition is frequently lethal, despite thrombolysis.1,2 Adjunctive catheter fragmentation may prevent death.1-3 However, commercial systems for fragmentation of thromboembolic material are not widely available, and reported experience with these techniques is limited.
In cases of major pulmonary embolism, patients are at serious risk of death due to right ventricular failure within the first hour of onset.4 Survival depends on rapid recanalisation of the pulmonary arterial occlusion and reduction of the right ventricular afterload. According to the results from a multicentre registry, overall in-hospital mortality rate ranges from 25% for patients presenting with cardiogenic shock to 65% for patients undergoing cardiopulmonary resuscitation.2 Thrombolytic therapy is a useful adjunct to heparin in patients who have pulmonary embolism and who are haemodynamically unstable.5 Rapid improvement of right ventricular function and pulmonary perfusion, accomplished with thrombolytic therapy followed by heparin, may lead to a lower rate of death and recurrent pulmonary embolism.1,5 However, in severe cases, even high-dose thrombolytic therapy may not prevent death.2 Transvenous catheter embolectomy or open surgical embolectomy should be considered in patients for whom thrombolysis is contra-indicated or deemed unsuccessful.3,6
Greenfield et al introduced the first percutaneous catheter thrombectomy device, an aspiration catheter, in 1969.7 There are currently several catheter thrombectomy techniques: aspiration thrombectomy, fragmentation thrombectomy, and rheolytic thrombectomy.3,6-8 However, the commercial devices used in these procedures are not widely available, and there is limited experience reported with any of these techniques. This type of procedure is typically confined to major interventional laboratories with experienced operators.
There are several reports of successful fragmentation of pulmonary emboli with improvised equipment in patients with shock.9 Our case is of particular interest, as thrombolysis had failed to improve the patient’s immediate clinical state. The use of a pulmonary flotation catheter to dislodge the embolism from the main pulmonary artery proved to be a useful temporising measure. This type of catheter is easily passed without the need for fluoroscopic control. In this situation, transoesophageal echocardiography proved pivotal in diagnosing the embolism rapidly, and in monitoring the response to therapeutic manoeuvres.10 Movement of the embolism from the main pulmonary artery was visualised in real time, which may be better achieved with transoesophageal rather than transthoracic imaging. Further mechanical fragmentation could then be achieved in the catheterisation laboratory. The technique consists of fragmentation of central emboli and dislocation of the fragments to the periphery, resulting in a relative gain of non-obstructed, cross-sectional artery area. Moreover, the increased total surface area of the fragments may ac-celerate the efficacy of concurrent thrombolysis.
In summary, for patients with major pulmonary embolism for whom thrombolysis is contraindicated or unsuccessful, the passage of a pulmonary flotation catheter and improvised catheter fragmentation of thrombus may be considered if there is suitable access to an interventional laboratory and an experienced interventionist.
- 1. Guidelines on diagnosis and management of acute pulmonary embolism. Task Force on Pulmonary Embolism, European Society of Cardiology. Eur Heart J 2000; 21: 1301-1336.
- 2. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997; 30: 1165-1171.
- 3. Goldhaber SZ. Integration of catheter thrombectomy into our armamentarium to treat acute pulmonary embolism. Chest 1998; 114: 1237-1238.
- 4. Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis 1975; 17: 259-270.
- 5. Goldhaber SZ. Thrombolysis for pulmonary embolism. N Engl J Med 2002; 347: 1131-1132.
- 6. Schmitz-Rode T, Janssens U, Duda SH, et al. Massive pulmonary embolism: percutaneous emergency treatment by pigtail rotation catheter. J Am Coll Cardiol 2000; 36: 375-380.
- 7. Greenfield LJ, Proctor MC, Williams DM, Wakefield TW. Long-term experience with transvenous catheter pulmonary embolectomy. J Vasc Surg 1993; 18: 450-457.
- 8. Koning R, Cribier A, Gerber L, et al. A new treatment for severe pulmonary embolism: percutaneous rheolytic thrombectomy. Circulation 1997; 96: 2498-2500.
- 9. Murphy JM, Mulvihill N, Mulcahy D, et al. Percutaneous catheter and guidewire fragmentation with local administration of recombinant tissue plasminogen activator as a treatment for massive pulmonary embolism. Eur Radiol 1999; 9: 959-964.
- 10. Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med 2002; 136: 691-700.





None identified.